In this episode, Dr. Debra Kimless and Steve Goldner share their knowledge on the science, policy, and market evolution of medicinal cannabis. We start with the differences between THC and CBD, how they work in the body, and how they act on the brain. We discuss the many potential benefits of using CBD, THC, hemp in the various forms of administration (smoking, vaping, edibles, oils, etc.) as well as some of the safety issues including the recent uptick in incidents of hospitalization and death linked to vaping. Debra and Steve are both involved with the company, Pure Green—Debra the Chief Medical Officer and Steve the founder and CEO—whose aim is to create the safest, most efficacious form of delivery of cannabis. Their bigger mission is to shift the perception of the cannabis plant, garner acceptance of its medicinal benefits, and ultimately get it descheduled on a federal level so more people can access cannabis for a range of chronic ailments.
Subscribe on: APPLE PODCASTS | RSS | GOOGLE | OVERCAST | STITCHER
We discuss:
- Debra and Steve’s background reason for their interest in medical cannabis [7:00];
- The history of medical use of cannabis [11:15];
- How THC, CBD, and other cannabinoids work [16:00];
- Hemp—What it is, special uses, and the 2018 farm bill [22:45];
- The legal status of CBD, Deb and Steve’s clinical trial, and how CBD differs from THC [30:15];
- The safety profile of THC [35:00];
- Is marijuana as a gateway drug? [45:30];
- Smoking vs. vaping vs. edibles—Benefits, risks, and mechanistic differences [53:30];
- Can you build up a tolerance to the effects of THC? [1:15:00];
- What do people generally want to get from using marijuana? [1:17:15];
- Cannabinoid synthetics [1:22:30];
- Efficacy of CBD oils as a sleep aid [1:25:00];
- Pure Green Cannabis [1:30:30];
- Anecdotal evidence and managing the hype surrounding cannabis in medical treatment [1:38:45];
- Aspirations for the future of medicinal cannabis, and the legal challenges that await them [1:45:15];
- Descheduling cannabis: A human rights issue [2:04:00] and;
- More.
Debra and Steve’s personal motivation in medical cannabis research [7:00]
- Debra started to consider non-pharmaceutical options as a pain reliever when her mother was dying from a pharmaceutical drug complication
- From 2013, she has investigated cannabis as a medicinal molecule
- She treats patients with cannabinoid medicines free of charge, sharing her findings with colleagues
- Steve began his career formulating methadone for Vietnam War veterans, who returned addicted to drugs
- He now develops different cannabis derived formulations to help people with PTSD and pain management
- Became interested in cannabis as a medicine 45 years ago to address PTSD symptoms
- Noticed that his friend with PTSD felt better when he smoked cannabis and realized it could be a medicine but was not (yet) legal
- Vowed to develop the cannabis medical and research space after his friend died of liver cirrhosis seven years ago
“… developing methadone at the beginning of my career was a first bookend and now developing all these formulations to help people with cannabis is the second book end on my career” – Steve Goldner
- The two met at IACM two years ago and now work together in venture (Pure Green Canna) to create the safest and most effective system to deliver medical cannabis
The history of medical use of cannabis [11:15]
“..it’s not a new medication. In Asia, the king fancied himself a pharmacist and he was treating people for all sorts of disorders, from gout to absent memory…The ancient Greeks used it topically on their horses, which is why I do know that the acid forms of cannabis medicine actually work because they love their warhorses. They applied the cannabis leaves to the horses bodies to reduce swelling, inflammation, and infection after a war or a battle” – Dr. Debra Kimless, M.D.
- Cannabis has been used as a medicine for over 5,000 years
- Leaves – with small amounts of trichomes– were applied to horses in order to relieve pain
- Indian use would mix cannabinoids with heated milk – called Bhang – which was consumed orally as an anesthetic
- The medicinal plant then became associated with drug policy around the 1930s
- Political inclination towards controlling people’s behaviors around drinking and recreational drug
- Marijuana became associated with THC intoxication and smoking
- The case against marijuana: led by the Nixon administration and propelled as part of the United Nations (UN) War on Drugs
- DEA designated it as a Schedule I (“no accepted medicinal use”) narcotic drug
- People who utilized marijuana became disenfranchised
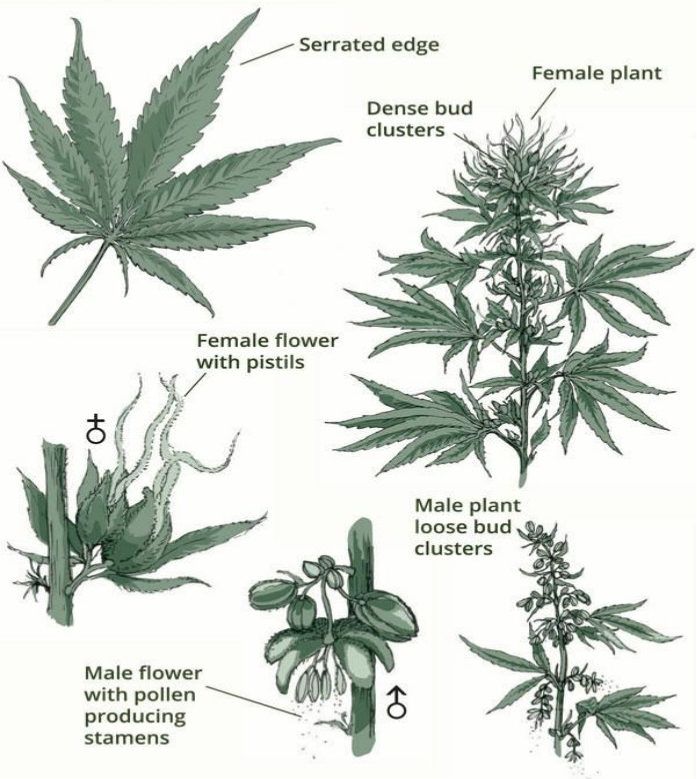
How THC, CBD, and other cannabinoids work [16:00]
“when it grows in nature, it grows as a flower. The leaf does have some cannabinoids in it or some of those medicines in it, but really the most bang for your buck, it comes from the flower where that’s what it is” – Dr. Debra Kimless, M.D.
- Grows as a flower in nature
- The leaf has some cannabinoids in it but most medicine is derived from the flower
Figure 1. The cannabis plant. Image Credit: curealeaf.com
“The interesting thing is that you can eat the cannabis plant all day long. You may get a bellyache from all the fiber, but you won’t get that intoxicating feeling because its chemical constituents are not in a way that can get you intoxicated” – Dr. Debra Kimless, M.D.
- Can eat the plant without intoxication …
{end of show notes preview}

Debra Kimless, Ph.D. & Steve Goldner, J.D.
Dr. Debra Kimless is a board-certified anesthesiologist with a certification in pain medicine who is best known for her strong provider-side advocacy of medical cannabis. She became interested in medical marijuana because her mother suffered from constant pain that did not respond to opiates. She traveled to both Israel and the Netherlands to study their well-regarded medical marijuana practice models. As the CMO of Pure Green, she conducts clinical trials of the company’s sublingual tablets.
Her goal is to de-stigmatize medical cannabis and educate both physicians and the public about its benefits. She is a frequent presenter at conferences and sees individual patients pro bono to develop treatment plans utilizing cannabis. She is also a “holistic” health proponent who combines her advocacy for medical cannabis with recommendations for a whole food, plant-based diet.
His focus is on drug-related investments with cannabis as a special area of interest. In addition to Pure Green, he runs 3 other businesses: Pinnacle Lab, which tests the safety/efficacy of marijuana; C3, a consulting firm for cannabis-based businesses; and Regulatory Affairs Associates, which helps companies with the drug and medical advice approval processes.
He advises NIH Phase 2 grant awardees through the FDA approval process, provides a free service to match patients to clinical trials, and holds several cannabis-related patents. He is also the Chairman of the USA Cannabis Laboratory Standards Setting Committee.
Steve Goldner is both a chemist and a lawyer but is probably best described as a medical entrepreneur. He is known as much for his business expertise as for his knowledge of pharmaceuticals or FDA law. In the 1970s, he co-invented both liquid methadone and urine drug screening testing, and early in his career he worked in FDA / regulatory affairs for several pharmaceutical companies. He is known for his expertise in the pharmaceutical industry in general and his knowledge of the FDA approval process in particular.
His focus is on drug-related investments with cannabis as a special area of interest. In addition to Pure Green, he runs 3 other businesses: Pinnacle Lab, which tests the safety/efficacy of marijuana; C3, a consulting firm for cannabis-based businesses; and Regulatory Affairs Associates, which helps companies with the drug and medical advice approval processes.
He advises NIH Phase 2 grant awardees through the FDA approval process, provides a free service to match patients to clinical trials, and holds several cannabis-related patents. He is also the Chairman of the USA Cannabis Laboratory Standards Setting Committee.
Website: stevegoldner.com






Regarding the impact of THC on sleep architecture, I seem to recall that your guest Mathew Walker, Ph.D, stated that THC inhibits REM — presumably not good. Would appreciate clarification of this issue.
I reviewed my notes from Matt’s podcast series and he did indeed indicate that THC was correlated with some inhibition of REM. I’m guessing we’ll simply have to wait for more studies to be conducted testing the effects on sleep architecture. I’ve included my notes from Matt’s podcast about THC and CBD below:
THC
-Does speed up time to fall asleep (sleep onset latency)
-However, THC seems to block REM sleep
-Dependency can occur, resulting in “rebound insomnia” when quitting
CBD
-Shows much more promise than THC
-Look for higher dose with mostly pure CBD (very little THC)
-May reduce core body temperature (hypothermic)
-May be anxiogenic (quiets the sympathetic nervous system); however, alcohol and THC also have anxiogenic effects, so this may be a case where the positives just happen to slightly overshadow the negatives
It seems like it was some skewed interpretation by Mathew Walker of experimental data showing a decrease of REM signs in EEG preceded by THC. Technically, such decrease in REM doesn’t 100% mean that it is “suppressed” or “blocked”, it can as well be just in another mode of operation – in humans. At least dr. Walker didn’t mention
any plausible cellular mechanisms for such “suppression” by THC on REM-generating centers in the brain stem. This is what happens when there is too much reliance on rat data, disguising the fundamental impotency to perform maximally ecologically valid and minimally invasive measurement of 80% of crucial cellular activity in humans in vivo.
Hi Peter
Can I recommend Dr Ethan Russo’s work on the often under-appreciated benefits of the terpenes. I won’t paste the link here but a YouTube search for: “The Pharmacology of Cannabis, Cannabinoids and Terpenes by Dr. Ethan Russo” will get anyone to where they need to be.
He does an excellent job of going into the terpenes which are often glossed over but clearly are part of the multi-modal effect that was talked about in your excellent conversation.
Wonderful podcast with great value information, thank you for doing this Peter!
I hear that you discuss vape cartridges with cannabis oil and what Steve said is logical and i consider correct. But what about vaping the pure herbal material with a vaporiser on a low temperature? For example i do it in 170 which i find very calming and nice. I think THC goes out at 180 so i assume what i’m getting is the CBD only? Do you have any research on that?
It is rather illogical (per logic of ample obsessive medical thought regarding longevity) that after examining the question of “Why people can be addicted to THC?” Peter Attia didn’t examine the question of “Why people are way more addicted to having breakfast, dinner and supper every day?” and didn’t examine the risks of decreased autophagy, decreased ketosis, decreased stem cell activation, etc modulated by regular not skipping 1-3 of them.