In a word, yes. But, technically this is the wrong question.
The correct question is probably closer to, “What is the impact of the calories I consume on my body’s ability to store fat versus burn fat?”
The immediate follow-up question to some variant of this first question is, “Should I be counting calories?” In a word, no. But you’ll want to read this post fully to qualify that answer.
Before I answer these important questions, let’s spend a few moments reviewing five key concepts.
Key concept #1 – the definition of a calorie
A calorie is a unit of measurement for energy content. By formal definition a calorie is the amount of heat energy required to raise one gram of water from 14.5 to 15.5 degrees Celsius at atmospheric pressure. One-thousand calories is equal to 1 kilocalorie, or 1 kcal for short. Here’s where it gets a bit tricky. Most people use the term “kilocalorie” and “calorie” interchangeably. So when someone says, “a gram of fat has 9 calories,” they actually mean 9 kcals. The important thing to remember is that a calorie (or kcal) tells you how much energy you get by burning the food. Literally. In the “old days” this is how folks figured out the energy content of food using a device called a calorimeter. In fact, to this day this is how caloric content is measured when doing very precise measurements of food intake for rigorous scientific studies. As a general rule carbohydrates contain between 3 and 4 kcal per gram; proteins are about the same; fats contain approximately 9 kcal per gram.
[If you’re wondering why fats contain more heat energy than carbohydrates or proteins, it has to do with the number of high energy bonds they contain. Fats are primarily made up of carbon-hydrogen and carbon-carbon bonds, which have the most stored energy. Carbs and proteins have these bonds also but “dilute” their heat energy with less energy-dense bonds involving oxygen and nitrogen.]
Key concept #2 – thermodynamics primer
It might be a good time, if you haven’t done so recently, to give a quick skim to my previous post, revisit the causality of obesity. In this post I review, among other things, how the First Law of Thermodynamics explains fat accumulation and loss. To reiterate, the First Law of Thermodynamics says that the change in energy of a closed system is equal to the energy entering the system less the energy leaving the system. When we apply this to fat accumulation, it looks like this:
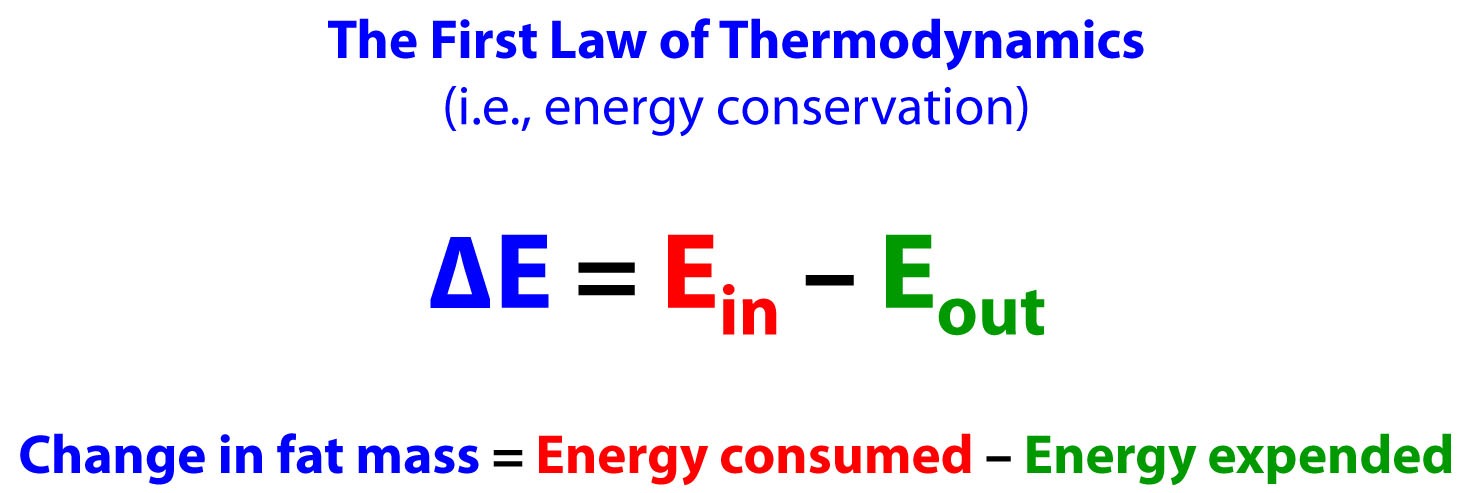
People like me (and others) get a bad rap from folks who lack the patience (or training, perhaps) to actually hear the entire argument through before throwing their hands in the air, waving them frantically, and screaming that we’re violating the First Law of Thermodynamics for asserting the Alternative Hypothesis (more on this below).
Let me be as crystal clear as possible, lest anyone feel the need to accuse me of suggesting the Earth is flat. The First Law of Thermodynamics is not being violated by anything I am about to explain, including the Alternative Hypothesis.
Key concept #3 – current dogma
Conventional wisdom, perhaps better referred to as Current Dogma, says that you gain weight because you eat more than you expend. This is almost true! To be 100% true, it would read: when you gain weight, it is the case that you have necessarily eaten more than you expended. Do you see the difference? It’s subtle but very important — arguably more important than any other sentence I will write. The first statement says over-eating caused you to get fat. The second one says if you got fat, you overate, but the possibility remains that another factor led to you to overeat.
If you believe Current Dogma, of course you’ll believe that “calories count” and that counting them (and minimizing them) is the only way to lose weight.
Key concept #4 – the rub
Most folks — but not all — who subscribe to Current Dogma do so, in part, because they don’t appreciate one very important nuance. In the equation above, explaining the First Law of Thermodynamics, they assume the variables on the right hand of the equal sign are INDEPENDENT variables.
Let me explain the difference between independent and dependent variables for those of you trying to suppress any memories you once had of eigenvectors. As their names suggest, independent variables can change without affecting each other, while the opposite is true for dependent variables. A few examples, however, are worth the time to make this easy to understand.
- The weather and my mood are dependent variables. When the weather goes from gloomy to sunny my mood tends to improve as a result of it, and vice versa (i.e., when the weather goes from sunny to gloomy, my mood goes from good to bad). In this case the dependence is only one-way, though; my mood changing has no impact on the weather.
- My countenance and my interaction with people are dependent variables. When I smile it seems to cause a more positive interaction with the people around me. Similarly, when I’m having a good interaction with someone I tend to smile more. In this case the dependence goes both ways.
- My height (while I was still growing) and my hair length are independent variables. Both of these variables can change without any impact on each other.
How does this tie into the idea of the First Law? Let’s re-write the First Law with a bit more specificity:
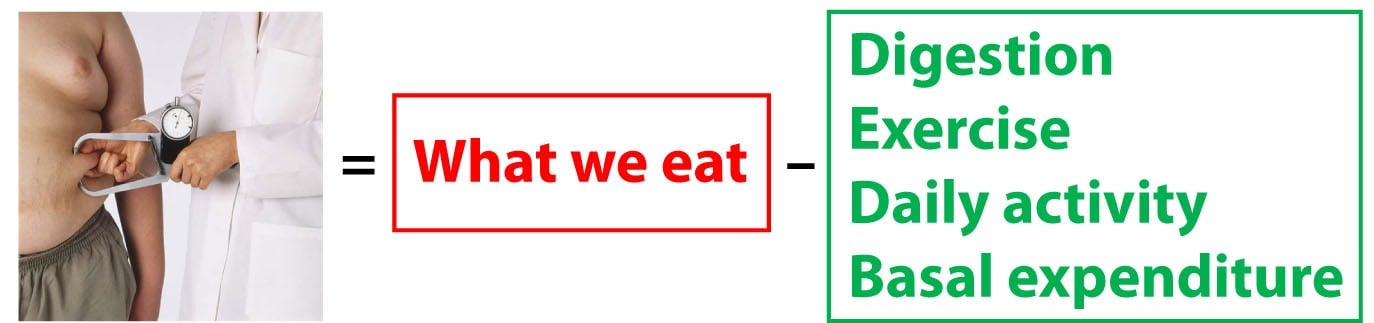
The change in our fat mass is equal to what we eat and drink (the only source of energy entering our system) less all of the energy we expend.

Now let’s be even more specific on the “expend” part of the equation. We expend energy in four ways: Digestion (all the energy we require to break down food, plus the undigested portions that leave our body); Exercise (everyone knows what this is, but I tend to separate it from daily activity since people really like to focus on exercise); Daily activity (the non-exercise activity we carry out); Basal expenditure (the energy we expend “underlying” any activity – e.g., when you are resting).
Let me clarify something before going further. There are several ways to enumerate and account for our energy expenditure. I happen to do it this way, but you can do it other ways. The important thing is to make sure that you are collectively exhaustive when doing so (and mutually exclusive if you want to make your life easier – we call this MECE, pronounced “mee-see”).
The First Law is only valid when you consider ALL of the energy entering and leaving the system (i.e., your body).
Back to the independence versus dependence issue for a moment. If you look at the equation above, and believe the red box has no impact on the green box, and vice versa, you are saying that energy input and energy expenditure are independent variables. However, this is not the case, and that is exactly why this problem of energy balance is so vexing. In fact, the figure below is a more accurate representation of what is actually going on (and even this is a gross oversimplification for reasons I will mention shortly).
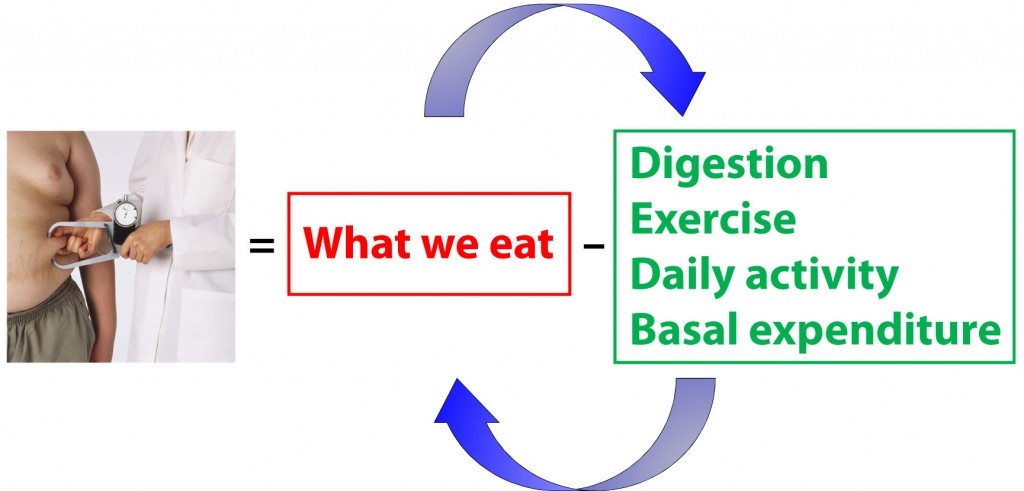
What you eat actually changes how you expend energy. Similarly, how you expend energy changes what (and how) you eat. To be even more nuanced, what you eat further impacts what you subsequently eat. As you increase (or decrease) in size, this impacts how you expend energy.
So there are actually a whole bunch of arrows all over this diagram (I’ve only shown 2: what you eat impacting how you expend, and vice versa. If I included all of the arrows, the diagram would get out of control pretty quickly).
I’m not telling you anything you don’t already know, even though it may sound like it for a moment. When you exercise your appetite rises relative to when you don’t exercise. When you eat a high carb meal you are more likely to eat again sooner compared to when you eat a high fat/protein meal due to less satiety.
Key concept #5 – the Alternative Hypothesis
If, like me, you don’t subscribe to Current Dogma, you’d better at least have an alternative hypothesis for how the world works. Here it is:
Obesity is a growth disorder just like any other growth disorder. Specifically, obesity is a disorder of excess fat accumulation. Fat accumulation is determined not by the balance of calories consumed and expended but by the effect of specific nutrients on the hormonal regulation of fat metabolism. Obesity is a condition where the body prioritizes the storage of fat rather than the utilization of fat.
Why is this different from Current Dogma? Current Dogma says it doesn’t matter what you eat, it only matters how many calories that food contains. If you eat more calories than you expend, you gain weight. The last part is true, but the first part is not. The Alternative Hypothesis says it DOES matter what you eat and for reasons far beyond the stored heat energy in the food (i.e., the number of calories).
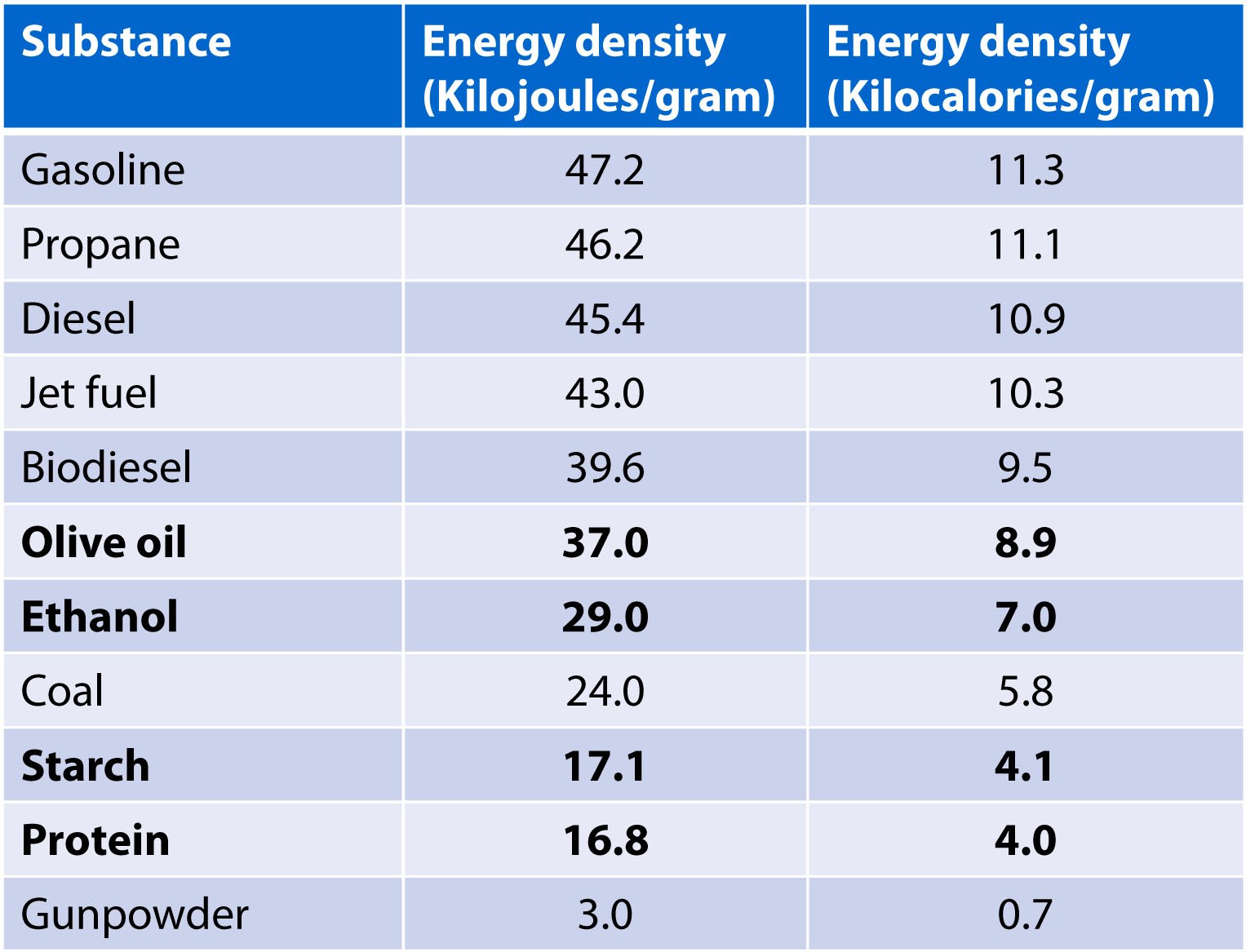
Let me use an example to illustrate this. Consider the following table of various substances known to contain a lot of stored energy. The table shows their energy content in units we usually use to describe energy density, kilojoules per gram (middle column), and I’ve converted to units we typically only use for food energy, kcal/g or “calories” per gram, (right column). [Here we need to be very clear to distinguish between a technical calorie and a kilocalorie, which is almost always what we mean.] A kilojoule is about 240 calories (not kilocal), so 1 kj is about 0.24 kcal, and therefore 1 kj/g is about 0.24 kcal/g.
I’ve highlighted, in bold, four rows of things we typically eat: fat (olive oil, to be specific) with about 8.9 kcal/g; ethanol with about 7.0 kcal/g; starch with about 4.1 kcal/g; and protein with about 4.0 kcal/g.
I’ve also included in this table some other substances known to contain chemical energy such as liquid fuels (e.g., gasoline, diesel, jet fuel), coal, and gunpowder. Hard to imagine a world without these chemicals, for sure.
A quick glance of the table, which I’ve ordered from top to bottom in terms of caloric density, would suggest eating olive oil would be more “fattening” than eating starch since it contains more calories per gram, assuming you subscribe to Current Dogma.
But that same logic would also suggest eating coal would be more fattening than starch and gunpowder less fattening than ethanol. Gasoline would be more fattening than jet fuel. Hmmmm. Anyone interested in testing this hypothesis (personally)? Despite my wildest self-experiments, this is one self-experiment I’ll pass on. Why? Well for the same reason you’d pass on it – you know that there are far more important consequences to drinking diesel or snorting gunpowder than their relative energy densities.
Sure, everything on this list is an organic molecule largely composed of the following four atoms: carbon, hydrogen, oxygen, and nitrogen. Not to bore everyone with a lesson on organic chemistry, but it’s the actual bonds between these atoms that are responsible for their energy densities. When you “liberate” (i.e., break) the bond between an atom of carbon and hydrogen, for example, you release an enormous amount of stored chemical energy. This table tells you exactly how much energy you would release if you were to break the bonds in these molecules, but that’s all it tells you. You can’t actually know, just by looking at this table, if jet fuel is more paraffinic than diesel or if gasoline has more isomerization than propane. And, you certainly have no idea, from the information contained in this table, of exactly how each of these substances will impact the hormones, enzymes, and cell membranes in your body if you ingest them.
Is it relevant to our bodies that olive oil has about the same energy density (i.e., calories) as biodiesel (also known as fatty-acid methyl-ester)? Or, is it more relevant to us that consuming olive oil has a very different effect on our bodies than consuming biodiesel beyond anything to do with the calories contained within them? Obviously consuming equal caloric amounts of olive oil versus biodiesel will have a very different impact on our body. Why then is it so hard to appreciate or accept that equal caloric values of olive oil and rice could also have very different impacts on our body?
The upshot
Let’s get back to the question you actually want to know the answer to. Do calories “matter”, and should you be counting them?
Energy density (calories) of food does matter, for sure, but what matters much more is what that food does in and to our bodies. Will the calories we consume create an environment in our bodies where we want to consume more energy than we expend? Will the calories we consume create an environment in which our bodies prefer to store excess nutrients as fat rather than mobilize fat? These are the choices we make every time we put something in our mouth.
Our bodies are complex and dynamic systems with more feedback loops than even the most elaborate Tianhe-1A computer. This means that two people can eat the exact same things and do the exact same amount of exercise and yet store different amounts of fat. Does it mean they have violated the First Law of Thermodynamics? Of course not.
Similarly, genetically identical twins can eat different macronutrient diets (i.e., differing amounts of fat, protein, carbohydrates) of the same number of calories, while doing a constant amount of exercise, and accumulate different amounts of fat. Does this violate the First Law of Thermodynamics? Nope.
What you eat (along with other factors, like your genetic makeup, of course) impacts how your body partitions and stores fat. In case anyone is wondering how I got over 2,000 words into this post without mentioning the i-word, wonder no longer. Insulin, while not the only factor involved in this process, is probably at the top of the list. When you eat foods that have the double whammy of increasing insulin levels AND increasing your cell’s resistance to insulin, your body prioritizes fat storage over fat utilization. No one disputes that insulin is the most singularly important hormone for causing fat cells to accumulate fat. Somehow the dispute centers on what causes people (full of billions of fat cells) to accumulate fat.
All calories are not created equally: The energy content of food (calories) matters, but it is less important than the metabolic effect of food on our body.
Photo by Aaron Barnaby on Unsplash




Hi Peter. Thanks for the great site. I have been on a ketogenic diet for 5 months and I have lost 70 lbs (although I have about 140 more to go – I started at 345 lb). When I first started, I was eating around 1800 calories a day, with the number of daily calories steadily declining to where I now struggle to eat 1000 calories per day and some days 800. Throughout, no matter the number of calories I consume, I always end up with a macro ratio of 65-70% fat, 25-30% protein, and 2-5% carbs. My concern right now is that I am not eating enough calories, although I do manage to keep my protein to within 70-90 g. Since I am in ketosis, where I have access to my own body fat to burn for fuel, do I need to worry about eating a certain number of calories (i.e. at least 1200) to keep my metabolism ticking along, or does this only apply to carb-burners? Do I need to try to force myself to eat more, or just eat when I am hungry (in which case I will probably eat around 1000 cals)? Who knew that not eating enough and rarely being hungry would ever be a “problem” that I would have to worry about 🙂
Crystal, as crazy as this sounds, you are eating more than you know. If you’ve lost 70 pounds in 5 months, and assuming about 10 pounds of that was water, it means you’re averaging about 180 gm/day of fat loss (net). This is much more than most. Average is about 65 gm/day and up to about 130 gm/day. What this means, of course, is that your body is “eating” itself to the tune of about 1,600 kcal/day. So with your intake at 1,000 kcal/day, this suggests your body is requiring about 2,600 kcal/day per day: 1,000 from what you eat, and 1,600 from your own fat stores.
Hi Peter,
First, I think this post and your blog are absolutely brilliant. I’ve read some of Gary Taubes’ work as well, and it totally rocked my world.
Anyway, I was wondering if you could help me work out a little mystery.
I am 24 years old, female, 5′ 4” and for most of my teenage/adult life, I tended to be chubby. I never ‘exercised’ exactly (though I wasn’t totally sedentary either– I would walk to classes and around town w/ friends, etc) and I ate whatever I felt like, which included a lot of fast food, junk, and carbs. My weight pretty much stuck between 130-145. Once, it even went all the way up to 158 (perhaps due to a change in medications).
About two and half years ago, I got sick of feeling chubby, and finally, for the first time, tried to seriously diet. I did the south beach thing, and started working out, doing about an hour of yoga a day and speed walking for 30 minutes. I was eating around 1500 calories, and I lost a ton of weight. It all sort of spiraled into what I recognize now as an eating disorder (I was going through a lot of other emotional issues at the time). I was down to 106 pounds, calorie counting obsessively, and had intense anxiety over food and exercise. Eventually I realized I was no longer well, and tried to pull myself together and eat normally again. Luckily, because I had never suffered from these issues before, and because I had a supportive fiance, I was able to ‘snap out of it.’ It took a while for my anxieties to disappear, but I was able to start eating normally (probably even over-eating) relatively easily, because my body had been hungry for so long, I was craving food anyway.
That brings us to today. For a year or so, I have been consciously, purposefully eating as much as I want of whatever I want– which is to say, probably poorly. I am back to eating sweets and carbs– which have always been my weakness. I am, perhaps, a little more active than I used to be, but I am back to exercising infrequently– sometimes I will go weeks without doing anything more strenuous than just walking around. And the calorie-counter in me KNOWS that I must be consistently eating more than I expend– I counted calories neurotically for a year, so I can’t help but have a pretty good idea of how many calories I eat, even when I try not to think about it. And many of those calories are carbs.
And yet, (and here is the mystery), I have stabilized at about 115 pounds. It has been over a year now of near-inactivity and eating junk, and my weight has not fluctuated at all. A base weight of 115 is way thinner than I ever used to be, when I was eating the same number of calories, eating the same carbs, at the same general activity level. So what’s the deal here? The science doesn’t seem to add up– whether you subscribe to the calories in=calories out OR the carbs vs low carbs theories.
Did my eating disorder somehow reset my metabolism (instead of messing it up like everyone warned it would)? Must I just be hallucinating and eating less/excising more than I realize? Did I fix my bodies response to carbs? Does it have to do with getting older?
I’d be interested to hear any theory you might have– I have been really perplexed for a while!
Maia, there is so much I’d like to say (and speculate) about this, but I don’t know how to do so in short order. I will say this, at some point I will definitely address this in the context the relationship between obesity and anorexia (I’m not saying you have the latter, but you’ll understand why I’m making this point when I get to it). The issue is one of too much or too little fat storage vs. what most would say is a behavioral issue.
Peter,
I know you are an extremely busy man with so many topics you want to explore but I would be very interested on your take on obesity and anorexia versus behavioral issues. I’ve come to the conclusion that chemistry, biology , physiology trumps behavioral this time. I’ve gone the behavioral therapy route and it is not a solution or fix.
Of course try to explain that to the current believers in that and well.it doesn’t get one very far or gets one any real help.
I definitely look forward to addressing this. I believe anorexia may be as misunderstood as obesity.
Hi Peter!
Love your blog! Got a question, is there a minimal daily caloric intake an individual (lets take me for example 5ft7, 68kg girl, 20yrs old) has to meet to reach ketosis? The reason I’m asking this is because I often find it hard to get in more than 1500kcals a day and I had underactive thyroid issues in the past on a vegan diet and the last thing i wanna do here is to put my body in a starvation mode again which is what you do when not eating enough.However I find keto diet to be very satiating and often forget to eat and when I do I eat less (smaller meals that is) however I do keep it at 3 meals a day to boost my caloric intake…My macros are 70 to 80 percent fat, around 20 percent protein and less than 10 percent carbs…Moderate activity level, 30 to 45 min workout daily or every second day (mostly squats, lunges, leg raises, kb swings, etc)…So to make long story short is it only about getting the right macros or there is a calorie minimum one should satisfy to enter ketosis in first place? Also in that case how to differentiate keto adaptation weakness and fatigue from being too low in calories, any advice would be much appreciated! Much love!
MsMaar 🙂
No, in fact the fewer calories (e.g., starvation), the easier it typically is.
Hi Peter,
I first saw you on a very emotional video about metabolic syndrome – I’m sure you know the one. Very inspirational stuff, loved it!
Anyhow, bit of a nerdy question for you here…
I can see you are an advocate of the ketogenic method to body fat reduction…had you ever tried using a diet primarily made up of carbohydrates (small amounts) to use a pre-curser for gluconeogensis (& lipolysis) via the krebs/citric acid cycle as glucose—>pyruvic acid —>(fatty acids)—> Acetyl co-a —> ATP?
An alternative method which although supported by our biochemistry – is rarely seen practiced to my knowledge. Wondered if you had tried to reduce body fat consciously this way??
Thanks,
Phil.
Phil, for pyruvate to go to acetyl CoA to FA, requires a lot of carbohydrate. This is called de novo lipogenesis. See the post on fat flux for a greater explanation.
Thanks for the reply Peter.
As I understand, carbohydrate will not really suffer lipogenesis unless glycogen levels are at the maximum level right? And as far as there is glycolysis available, gluconeogenesis isn’t needed as a route to ATP, hence the key in the method above would be keeping glycogen levels very low – just enough to provode the pre curser for fat to enter the krebs cycle.
It’s a good discussion point for me – I used the method above to go from 28% to 10% in 5months wothout exercise and my diet consisted of 80% carbohydrate (most kinds), very little fat & very little protein. Essentially, the opposite of what you did for your transformation.
Hi Peter,
I am a 42 years old guy from Spain that has lost 55lbs in the last year. Moreover I feel better than ever (specially about my former NAFLD, high iron levels and hiatus hernia). I owe that to you and to Gary Taubes, because it were your videos on youtube what really helped me a few months ago.
Today I created a small document trying to put some of the ideas from your article in a more visual form. I am an engineer so please forgive any mistake in the document. I just wanted to test if it was a good idea to explain things more visually. I hope you like it.
https://www.mediafire.com/view/eq9nrsa3qpafhid/firstlaw.pdf
Your sincerely,
Vicente
Thanks very much for sharing, Vicente.
Let’s say I have a business: I sell only one model of car and the benefits I get every year can
be calculated as:
B=N*(P-C)-E
where N are the number of cars I sell in a year, P is the selling price of one car,
C is their unitary cost and E are my other expenses. If I ask a nutritionist how can I earn more money he/she could say “to earn more money you have to earn more money”. True, isn’t it? But useless, so I ask another one and he/she says:
“It’s very simple, you have to:
1) Raise the price of the cars
2) Sell more of them”
So, isn’t it true that you will earn more money if you sell more cars at a higher price?
Of course it is. Who can deny that?
But the questions are, a) can you really do that and b) do it without changing other terms of the equation,
short term or in the long run?
Can you really raise the price without reducing your sells?
Are you really going to sell more cars just because you have decided to? Even if you achieve to sell more
cars at a higher price, is it sustainable? How will your expenses change if you sell more cars? (can you sell
more without increasing the staff?) The answer is simple: an equation doesn’t give you the clues
to have a succesful business. You need to understand how the market works.
“Eat less and exercise more” is as useless as “sell more at a higher price”.
Very nice analogy, Vicente.
Yea…nice, clean, concise, simple. We Keto-Gypsies need stuff like that. I’m just enjoying reading these posts. Swift.
Hi Peter,
you wrote elsewhere in the blog that explanations on this topic are much longer than the “calories in, calories out” falacy. As you said, that is a handicap when trying to explain things.
I believe the following counterexample may be interesting:
For 3 weeks a guy eats 5700 Cals/day mainly FAT and his weight goes up 1Kg. His CVD risk factors get better.
For another 3 weeks the same guy eats 5700 Cals/day mainly CARBS and his weight goes up 7Kg. His CVD risk factors get worse.
How can the weight gain be completely different if he eats the same Cals/day in both experiments? How can it all be about the total calories you eat? Are all calories the same when talking about health if certain kinds of food make you sick and others don’t?
NOTE: This is not a thought experiment. The guy’s name is Sam Feltham and here you can find a complete report
https://live.smashthefat.com/why-i-did-get-fat/
I’m aware of Seth. I can’t speak to the accuracy of his calorie counting, “ins” and “outs,” etc. My point is that glycerol shortage may not be the reason L>R-E (see nomenclature from fat flux post), which is is the necessary condition to enable this.
Hi Peter,
you are right. It is a wise stance not to trust data unless there is a minimum warranty. That could be a false step.
I just liked the idea that it was simple to refute the “a calorie is a calorie” idea without too long explanations.
Apart from that, even if you in the future decided to stop publishing in your blog, you should know that what you already have done has been really important at least for me. You have provided a lot of people with the information needed to change our diets. When I told my wife I was going to have bacon for breakfast she got really really mad at me. When my friends knew I was eating atkins-style, they thought I was doing a stupid thing and asked me to visit a nutritionist. We need good info to resist all that social pressure. Moreover I didn’t know if I was able to exercise without carbs until I saw you on youtube saying you didn’t eat carbs when the duration of the exercise was less than 3 hours (if I don’t remember wrong). May be in the future NuSI can help change things for a lot of people, but you have already helped a few of us. I thank you for the time you invested writing your posts.
PD: now my wife also eats low-carb
This is a bit off the thread but I suspect it’s mostly about calorie restriction/starvation so I’m going to pop it in here. I was wondering if you had any thoughts on the juice detox craze, juicing and juicing fasts. I’m squarely with low carb myself and anything that involves that much fruit seems like it would necessarily be high in carbs, but the juicing proponents have a lot of claims about huge weight loss, improving health numbers and phytonutrients and micronutrients and the dangers of animal fat. They’ve got pretty good PR and well, they just make it all sound so healthful! I haven’t been able to find a low-carb counterpoint to the juicing claims. Just wondering if you had any thoughts on it. Thanks so much.
Hi Peter, love your work! Quick question. How can I optimize weight loss with my low/no carb plan that I just started 9 days ago?
Thanks very much from beautiful Vancouver BC Canada!!
Thanks very much,
Tony 🙂
I am not trained in medicine or biology, but everything I read about ketosis suggest a that a lot of stored eery is Elim ate via urination. Of ke tones are fact eliminated in this way, than shouldn’t they be included in your first law equation?
Peter,
Now that I am on a computer with a real keyboard instead of my smartphone, I will try to ask my question again.
Like I was trying to say in my first post, the research that I have done suggests that when a person is in a ketosis metabolic state a lot of stored energy in the form of ketones are excreted from your body through urine and breath. So should not this term be included in your first law equation? I know Dr Atkin’s book is fairly old, but his claim was that fat loss via his diet occurred because that a significant portion of the ketones formed from the breakdown of fat stores were peed away. Seems to work for me. I just finished reading Grain Brain by Dr. Perlmutter, which advocates a diet very similar to what Dr Atkins advocated. So last month I gave it a second try and so far have lost 30 pounds. So the results makes me a real believer. Eliminating the GLUTEN has reversed the arthritic pain that I had in my knees and fingers too. My stomach problem have also gone away.
Hi Steve,
I’m not Peter, and obviously I’m not in a position to answer for him.
However, somewhere on this blog, in a reply to a comment (I can’t find it now), I recall that he said that he had measured the amount of acetoacetone (“AcAc”), the principal ketone that is excreted in the urine, that he had excreted over a 24-hour period, and found it to be very small: I seem to recall, just a few calories’ worth, essentially meaningless. Basically, ketones are being used for energy (principally beta-hydroxybutyrate, “BHB”), not lost in the urine.
If you think about it, biologically the point of ketosis is to keep us alive by using the energy of our fat stores, not to waste that energy so we can lose weight :).
I remember the Atkins books, too, and for many years they provided my main understanding of ketosis. However, it looks like our present understanding has gone beyond what he was relying on. Peter and others have updated my information, and, frankly it makes more sense now.
I agree with you that ketosis is a good way to lose weight, and I have had positive experiences with it too. It just looks like the explanation is different, and perhaps more complex. (I’ll take results any day, with or without explanations….)
Bob.
Correct, it was less than 1 g in 24 hours, so only a few calories. That said, AcAc is not very stable, so it’s possible I excreted more, but only a small amount was measured. Second, because I was in ketosis for so long, it’s possible my body was much more efficient at using ketones, rather than excreting them, ergo, someone just entering ketosis may excrete more.
Dear Dr. Attia –
I believe wholeheartedly in the alternative hypothesis that you and Gary Taubes write about so persuasively. And I truly believe that the two of you (particularly through NuSI) will be changing medicine for the better to an extent that is unrivaled by anything I have seen in my lifetime. I feel seriously honored to be able to communicate with you.
But despite that suck-up intro, I have a bone to pick. Or maybe I’m missing the boat on something crucial here.
While I know the calories-in/calories-out hypothesis of weight gain is nonsense, it seems to me that there is a danger in going to far in the other extreme by suggesting that a calorie deficit isn’t important when trying to lose weight.
In other words, assuming an overweight person (me) is functioning well in ketosis for many weeks (which I have been for some time as shown by my O-OHB levels averaging 1.5-2.9 each day), isn’t it obvious that I will lose weight far more quickly/effectively if I take steps to ensure a caloric deficit?
I have been doing this by tracking my food intake on the Weight Watchers app. (As you know, the WW Points system is glorified calorie counting.) And the weight loss has been coming off quite quickly with zero hunger and cravings.
I lost nearly 40 pounds (almost all the weight I wanted) two years ago by following WW. But of course, since my nutrient composition didn’t put me in ketosis, this semi-starvation was difficult to follow. The weight came off too slowly for my taste. And of course I had yet another diet failure once I approached my goal. I could not have lived like that for any length of time.
My experience now is 180 degrees different. I am losing weight far more quickly than before, it is easy to stay within the prescribed amount of points, and I feel great. And I know for sure the only difference is the macronutrient composition of that I’m eating — which as I said keeps me “deeply” in nutritional ketosis.
I am not trying to endorse WW here. I know there are countless means of tracking caloric intake to ensure a caloric deficit.
I suppose some could argue that I would naturally eat the same number of calories that I am now so long as I remain in ketosis, but I don’t think so. I suspect I would eat enough to maintain my weight but not lose the average of 2.3 pounds that I have for the past month or two.
I believe that people who are seeking to lose weight via a LCHF diet/WOL/whatever would succeed much more frequently if they limited their calories too in a sensible manner rather than eat ad libitum. Imagine what the A TO Z study would have shown if the Atkins group also lowered their calorie intake.
Shouldn’t the message that one shouldn’t be counting calories (see intro to your post) be eliminated — at least as it relates to those trying to lose weight? (I suspect calorie counting will be entirely unnecessary for me once I’m at my goal weight, provided that I remain in ketosis.)
Or do you feel that my current weight loss rate would be identical even if I were eating ad libitum and consuming 500 more calories per day?
Thank you!
I should have said that I was losing a WEEKLY average of 2.3 pounds per week for the past month when following WW. This compares to about 1.0 pounds per week last time I was following WW.
ATTIA is a super guy in my never to be humble opinion. I’ve admired him for several years now. I just wanted to compliment you on your style re:….”despite that suck-up intro….” He has to dig it. In fact, with your tacit approval I’m going to use it myself someplace someday if you don’t mind. Very cool. Thanks. Take care.
Peter;
I have just found out about the low carb diet proposed on your website, The Eating Academy. Although I am trying to follow the diet, I must admit to a certain amount of confusion when it comes to calorie intake.
It’s easy to see, under your model of metabolism, why a body would not create new fat reserves even when consuming calories beyond it’s needs. Since there is so little insulin to drive that process, fat cells do not take up any excess. But what happens to the excesss? Is it simply excreted?
Further, if one’s intention is to lose weight, I fail to see how consuming calories in excess of the body’s need will allow this to occur. According to your model, no new fat will be deposited. But what mechanism would prompt the body to preferentially use fat reserves while sufficient, or even excess calories are presently being consumed? I have to be able to understand this.
Ibrom Evanston
I would also like to add: Thank you for putting this together. This is a great resource. You do a very great good here.
I don’t understand why Taubes, et al feel they need to be so cagey about this issue. Never a straight answer to this question. Still, after a ton of sleuthing, here is the answer. YOU MUST BE IN A CALORIE DEFICIT TO LOSE WEIGHT. There, that wasn’t so hard, was it? Yes, it matters tremendously if you are in ketosis. But even if you are in ketosis, you will not lose weight if you ingest calories surplus to what you expend.
Not sure what you mean about being cagey. I’ve never met a serious thinker on this topic who suggests weight loss doesn’t imply a caloric deficit. That’s a wonderfully descriptive fact that provides little explanation.
Peter, thank you for the courtesy of your reply. I am not trying to be simply argumentative here. Indeed, it is only due to Gary Taubes writing, and your website here that I have been able to take the first few steps in the long journey that is reclaiming my health and fitness. Still, when you state in the above article, “Should I be counting calories?” In a word, no.”, this confuses me. Because in order to metabolise my body’s stored fat, I do indeed need to be aware of how many calories my body is expending and how many calories I am consuming.
Thanks to you and Gary Taubes, I now have a much more fully nuanced understanding of how the food I eat affects my body, and this is crucial. But if the goal is reducing stored body fat, it seems like we come back around full circle to ‘calories in/calories out,” but with a greater depth of understanding of how differing macronutrients have different effects on the body.
Hey Ibrom,
When going Low Carb or Ketogenic after an adaptation period, your physiology changes to REDUCE hunger thus making it easier to eat less calories (negative caloric balance). So, ingestion of less calories is an EFFECT of the improved macronutrient profile. When eating a “balance” diet high in carbs it will tend to drive hunger up due to blood sugar and insulin fluctuations. If you believe the causal arrow goes in the other direction than you will be perpetually trying to cut calories below a certain level and most likely will be fighting against hunger, which is doomed to failure in the long run. There is no violation of the Laws of Thermodynamics. Its all about what direction you believe the CAUSAL arrow is pointing in the equation.
Paul
Peter:
This is a very interesting article. I particularly like how you turn the tables on the static version of calories in vs. calories out and the [correct] dynamic nature of the thermodynamics.
However, with the high level material you present in your posts, I seriously invite you to consider that you are wrong about the concept of bond energies as a “storage of energy” in molecules. This is really 101 of Chemistry! Breaking bonds always involves input of energy; there is no energy in the bond that can be “liberated”. It is the sum of
1) breaking bonds (consumes energy)
and
2) forming new bonds (makes energy available)
that decides if reactions are exothermic or endothermic. If 1) is larger than 2) the reactions are endothermic and if 2) is larger than 1) it is exothermic. Please, have a look at the following passages and compare them with any textbook or even wikipedia on chemical bond energies:
“[If you’re wondering why fats contain more heat energy than carbohydrates or proteins, it has to do with the number of high energy bonds they contain. Fats are primarily made up of carbon-hydrogen and carbon-carbon bonds, which have the most stored energy. Carbs and proteins have these bonds also but “dilute” their heat energy with less energy-dense bonds involving oxygen and nitrogen.]”
and
”Not to bore everyone with a lesson on organic chemistry, but it’s the actual bonds between these atoms that are responsible for their energy densities. When you “liberate” (i.e., break) the bond between an atom of carbon and hydrogen, for example, you release an enormous amount of stored chemical energy. This table tells you exactly how much energy you would release if you were to break the bonds in these molecules, but that’s all it tells you.”
It simply does not work that way!!! No energy is ”released” when breaking bonds, you have to put energy in. Hopefully, you have energy available in the system from bonds being formed, otherwise you will have to add heat or sometimes radiation.
Hey Magnus – I think this is where Dr. Attia was going with that statement about bond breaking.
As long as you accept that the oxidation of carbon fuels is a primary source of energy, and that energy is ultimately used to regenerate ATP from ADP and Pi (inorganic phosphate), it’s like this: the more reduced a carbon atom is, the more free energy is released upon its oxidation.
A free energy of oxidation (delta G) comparison of a single carbon compound will show this… Methane (-820 kJ/mol) vs. Methanol (-703 kJ/mol) vs. Formaledhyde (-523 kJ/mol) vs. Formic Acid (-285 kJ/mol) vs. Carbon dioxide (0 kJ/mol).
TL;DR: Carbons in fats are better sources of fuel than glucose because their carbons are more reduced (less oxidized). Just count the number of oxygens in a molecule of glucose and compare that to a fatty acid and my previous statement should make sense.
Nikolas:
I agree with your explanation of less oxidized carbons in pure hydrocarbon chains vs. partly oxidized CHO being the reason for differences in enthalpies of combustion.
Already formed C-O, C=O bonds means less energy available when forming new bonds.
The problem, however, is that I cannot make Peters wording fit with that.
Bonds are not energy storages! They are sinks. C-C are not as deep sinks as C-O. Still sinks, though!
For example this: “When you “liberate” (i.e., break) the bond between an atom of carbon and hydrogen, for example, you release an enormous amount of stored chemical energy.”
No energy is released at this step, on the contrary! The fact that you get energy from oxidizing H and C to water and CO2 at a later reaction step does not change this fact!
Hey Magnus:
I’d have to say that chemical bonds are indeed stores of energy, or sinks if you will. When you disrupt a chemical bond in the case of oxidizing our fuels (glucose or lipid) we are liberating/freeing the energy that was stored in that bond. Particularly, oxidation will allow H+ (hydrogen cations, protons) to create a Proton Motive Force (PMF) in the inner mitochondrial membrane where the Electron Transport Chain (ETC) passes electrons down their reduction potentials though cytochrome proteins to indeed synthesize ATP.
So I would say that, yes, energy is released in this series of steps that starts with a reduced fuel (hopefully lipid!) and ends with water and carbon dioxide. Energy input is required to make bonds and energy is given off when breaking them (think, positive or negative enthalpy… in relation to exothermic or endothermic reactions). Weaker bonds are broken to create stronger ones. Inspecting that Gibbs Free Energy (delta G) and Enthalpy (delta H) of the overall reactions of cellular respiration indeed should indicate to us that energy has been liberated from a broken chemical bond.
Dr. Attia, feel free to chime in if I am misunderstanding your wording. Likewise let me know if we’re still not in agreement, Magnus. 😀
Thanks for all the time and effort you put into these posts Peter. I have a question I’ve been wanting to ask and I think this is the correct place for it. In your “How I l Lost Weight” post you say “Two things jump out at you, I’m sure: I eat virtually all of my calories in the form of fat and my total caloric intake has actually gone up by about 50%. Let me reiterate, I don’t exercise any more today than I did 2 years ago. In fact, if anything, I probably exercise a bit less (i.e., down from 3-4 hours per day to 2 to 2.5 hours per day).” Aren’t you saying here that your energy expenditure went down, your energy intake went up, and during that time you lost weight? Sorry if I am misunderstanding this and thank you in advance if you can find the time to reply.
It appears in me that my non-deliberate EE went up. In my experience this effect is highly variable in people, and may also be transient.
After speaking with and interviewing Dr. Dominic D’Agostino I learned that energy expenditure does go up on a ketogenic diet. Some of the mechanisms he touched on included: activation of uncoupling proteins (thermogenin) in the mitochondria and sympathetic nervous system activation (catecholamines) which directly affect heart rate, blood pressure and body temperature.
Hope you find that as interesting as I did.
Peter, for those individuals who are insulin resistant (but not yet diabetic) or who for whatever reason are unable to lose weight despite restricting carbohydrates (and unable to reach ketosis without starving) for over two years, what options do they have other than to experiment with different caloric intake levels? Too few calories means slowing metabolism, too many calories means greater fat storage? I must confess that as I absorb your messages, I am left with a sense of despair because it seems impossible for me to create an environment where my body will achieve a negative fat balance, despite my best efforts to do so. This seems like a needle that can’t be threaded…
It’s slow going, but the shift is starting… albeit an odd piece for the Weather Channel.
https://www.weather.com/video/experts-eat-more-fat-54828
My friends and I are having this debate about the logic behind the 3500 calorie deficiency diet theory… Or whatever you want to call it. Anyways, the easiest basic explanation that I could figure out to explain why calories are not all created equal is to think in terms of a square and a rectangle. For example, to say that because 3500 calories equals 1lb of body fat then to create a 3500 calorie deficit means that you would lose 1 lb. is like saying that because all squares are rectangles means that all rectangles are squares. One truth does not always mean that the “opposite” is true as well. This article is a great explanation of what I am trying to get them to grasp and explains the concept very well.
Hi Dr. Attia,
To quote Richard Feynman on this topic from his book “The Character Of Physical Law” :
“When you hear of calories, you are NOT eating something called calories, that is simply the amount of heat energy that is in the food.”
This is in sharp contrast to the “fitness gurus” who always talk about “eating calories.” We eat no such thing.
These gurus do not realize that the human body derives its energy from the energy in the chemical bonds of the food we digest. This energy is then converted into heat and kinetic energy ( and thought) To write down any precise equation for the total energy balance of something as complicated as the human body would be extremely difficult
It is worthy to note calorie labels are wildly inaccurate- to the tune of 10 % to as much as 85% off ! – as Dr. Jeffrey M. Friedman ( from Rockefeller University) notes during his lectures. 🙂
Top biophysicists and physicists gave me some info 🙂
Best wishes,
Raz