In a word, yes. But, technically this is the wrong question.
The correct question is probably closer to, “What is the impact of the calories I consume on my body’s ability to store fat versus burn fat?”
The immediate follow-up question to some variant of this first question is, “Should I be counting calories?” In a word, no. But you’ll want to read this post fully to qualify that answer.
Before I answer these important questions, let’s spend a few moments reviewing five key concepts.
Key concept #1 – the definition of a calorie
A calorie is a unit of measurement for energy content. By formal definition a calorie is the amount of heat energy required to raise one gram of water from 14.5 to 15.5 degrees Celsius at atmospheric pressure. One-thousand calories is equal to 1 kilocalorie, or 1 kcal for short. Here’s where it gets a bit tricky. Most people use the term “kilocalorie” and “calorie” interchangeably. So when someone says, “a gram of fat has 9 calories,” they actually mean 9 kcals. The important thing to remember is that a calorie (or kcal) tells you how much energy you get by burning the food. Literally. In the “old days” this is how folks figured out the energy content of food using a device called a calorimeter. In fact, to this day this is how caloric content is measured when doing very precise measurements of food intake for rigorous scientific studies. As a general rule carbohydrates contain between 3 and 4 kcal per gram; proteins are about the same; fats contain approximately 9 kcal per gram.
[If you’re wondering why fats contain more heat energy than carbohydrates or proteins, it has to do with the number of high energy bonds they contain. Fats are primarily made up of carbon-hydrogen and carbon-carbon bonds, which have the most stored energy. Carbs and proteins have these bonds also but “dilute” their heat energy with less energy-dense bonds involving oxygen and nitrogen.]
Key concept #2 – thermodynamics primer
It might be a good time, if you haven’t done so recently, to give a quick skim to my previous post, revisit the causality of obesity. In this post I review, among other things, how the First Law of Thermodynamics explains fat accumulation and loss. To reiterate, the First Law of Thermodynamics says that the change in energy of a closed system is equal to the energy entering the system less the energy leaving the system. When we apply this to fat accumulation, it looks like this:
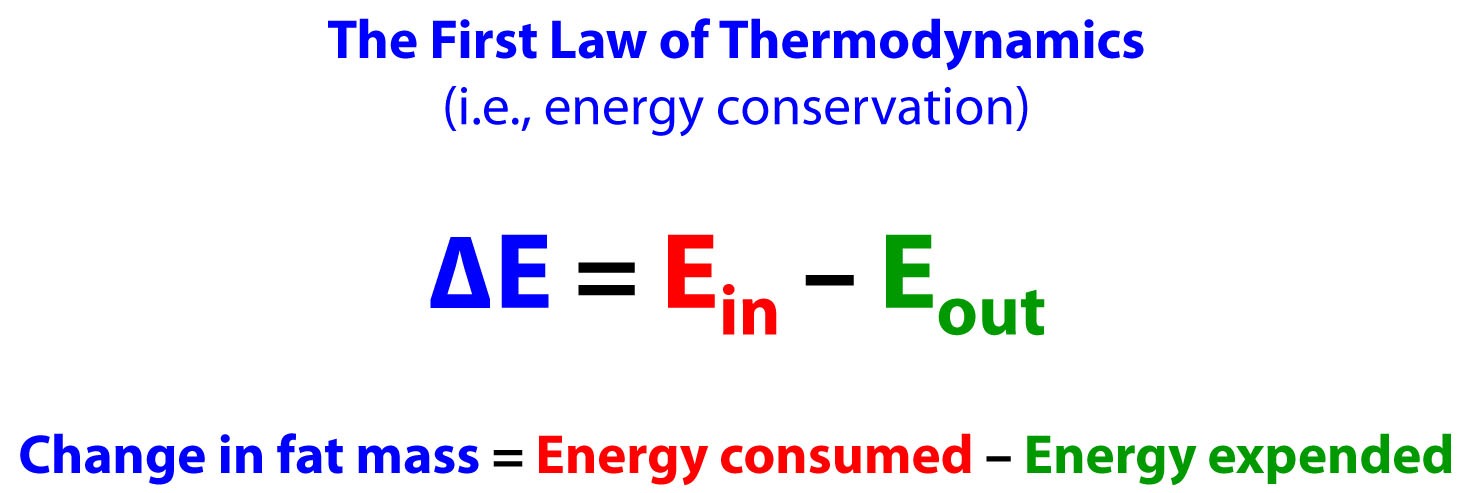
People like me (and others) get a bad rap from folks who lack the patience (or training, perhaps) to actually hear the entire argument through before throwing their hands in the air, waving them frantically, and screaming that we’re violating the First Law of Thermodynamics for asserting the Alternative Hypothesis (more on this below).
Let me be as crystal clear as possible, lest anyone feel the need to accuse me of suggesting the Earth is flat. The First Law of Thermodynamics is not being violated by anything I am about to explain, including the Alternative Hypothesis.
Key concept #3 – current dogma
Conventional wisdom, perhaps better referred to as Current Dogma, says that you gain weight because you eat more than you expend. This is almost true! To be 100% true, it would read: when you gain weight, it is the case that you have necessarily eaten more than you expended. Do you see the difference? It’s subtle but very important — arguably more important than any other sentence I will write. The first statement says over-eating caused you to get fat. The second one says if you got fat, you overate, but the possibility remains that another factor led to you to overeat.
If you believe Current Dogma, of course you’ll believe that “calories count” and that counting them (and minimizing them) is the only way to lose weight.
Key concept #4 – the rub
Most folks — but not all — who subscribe to Current Dogma do so, in part, because they don’t appreciate one very important nuance. In the equation above, explaining the First Law of Thermodynamics, they assume the variables on the right hand of the equal sign are INDEPENDENT variables.
Let me explain the difference between independent and dependent variables for those of you trying to suppress any memories you once had of eigenvectors. As their names suggest, independent variables can change without affecting each other, while the opposite is true for dependent variables. A few examples, however, are worth the time to make this easy to understand.
- The weather and my mood are dependent variables. When the weather goes from gloomy to sunny my mood tends to improve as a result of it, and vice versa (i.e., when the weather goes from sunny to gloomy, my mood goes from good to bad). In this case the dependence is only one-way, though; my mood changing has no impact on the weather.
- My countenance and my interaction with people are dependent variables. When I smile it seems to cause a more positive interaction with the people around me. Similarly, when I’m having a good interaction with someone I tend to smile more. In this case the dependence goes both ways.
- My height (while I was still growing) and my hair length are independent variables. Both of these variables can change without any impact on each other.
How does this tie into the idea of the First Law? Let’s re-write the First Law with a bit more specificity:
The change in our fat mass is equal to what we eat and drink (the only source of energy entering our system) less all of the energy we expend.

Now let’s be even more specific on the “expend” part of the equation. We expend energy in four ways: Digestion (all the energy we require to break down food, plus the undigested portions that leave our body); Exercise (everyone knows what this is, but I tend to separate it from daily activity since people really like to focus on exercise); Daily activity (the non-exercise activity we carry out); Basal expenditure (the energy we expend “underlying” any activity – e.g., when you are resting).
Let me clarify something before going further. There are several ways to enumerate and account for our energy expenditure. I happen to do it this way, but you can do it other ways. The important thing is to make sure that you are collectively exhaustive when doing so (and mutually exclusive if you want to make your life easier – we call this MECE, pronounced “mee-see”).
The First Law is only valid when you consider ALL of the energy entering and leaving the system (i.e., your body).
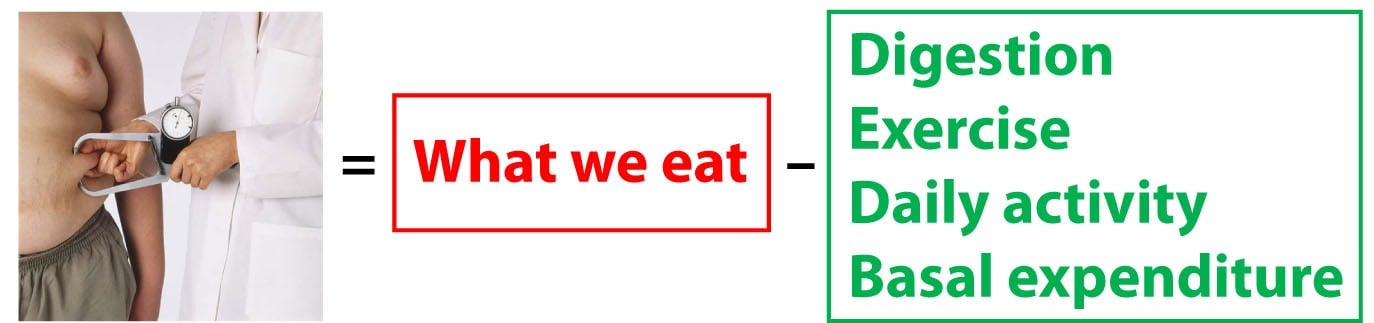
Back to the independence versus dependence issue for a moment. If you look at the equation above, and believe the red box has no impact on the green box, and vice versa, you are saying that energy input and energy expenditure are independent variables. However, this is not the case, and that is exactly why this problem of energy balance is so vexing. In fact, the figure below is a more accurate representation of what is actually going on (and even this is a gross oversimplification for reasons I will mention shortly).
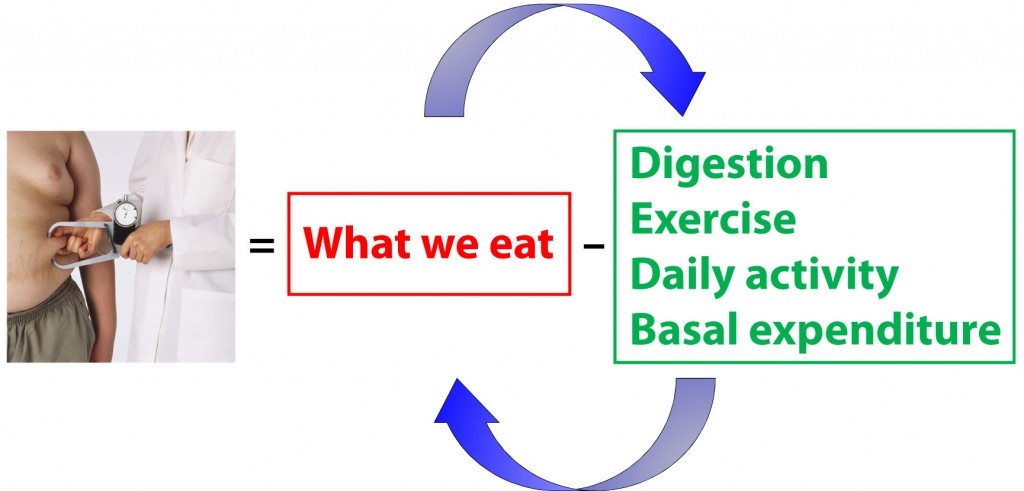
What you eat actually changes how you expend energy. Similarly, how you expend energy changes what (and how) you eat. To be even more nuanced, what you eat further impacts what you subsequently eat. As you increase (or decrease) in size, this impacts how you expend energy.
So there are actually a whole bunch of arrows all over this diagram (I’ve only shown 2: what you eat impacting how you expend, and vice versa. If I included all of the arrows, the diagram would get out of control pretty quickly).
I’m not telling you anything you don’t already know, even though it may sound like it for a moment. When you exercise your appetite rises relative to when you don’t exercise. When you eat a high carb meal you are more likely to eat again sooner compared to when you eat a high fat/protein meal due to less satiety.
Key concept #5 – the Alternative Hypothesis
If, like me, you don’t subscribe to Current Dogma, you’d better at least have an alternative hypothesis for how the world works. Here it is:
Obesity is a growth disorder just like any other growth disorder. Specifically, obesity is a disorder of excess fat accumulation. Fat accumulation is determined not by the balance of calories consumed and expended but by the effect of specific nutrients on the hormonal regulation of fat metabolism. Obesity is a condition where the body prioritizes the storage of fat rather than the utilization of fat.
Why is this different from Current Dogma? Current Dogma says it doesn’t matter what you eat, it only matters how many calories that food contains. If you eat more calories than you expend, you gain weight. The last part is true, but the first part is not. The Alternative Hypothesis says it DOES matter what you eat and for reasons far beyond the stored heat energy in the food (i.e., the number of calories).
Let me use an example to illustrate this. Consider the following table of various substances known to contain a lot of stored energy. The table shows their energy content in units we usually use to describe energy density, kilojoules per gram (middle column), and I’ve converted to units we typically only use for food energy, kcal/g or “calories” per gram, (right column). [Here we need to be very clear to distinguish between a technical calorie and a kilocalorie, which is almost always what we mean.] A kilojoule is about 240 calories (not kilocal), so 1 kj is about 0.24 kcal, and therefore 1 kj/g is about 0.24 kcal/g.
I’ve highlighted, in bold, four rows of things we typically eat: fat (olive oil, to be specific) with about 8.9 kcal/g; ethanol with about 7.0 kcal/g; starch with about 4.1 kcal/g; and protein with about 4.0 kcal/g.
I’ve also included in this table some other substances known to contain chemical energy such as liquid fuels (e.g., gasoline, diesel, jet fuel), coal, and gunpowder. Hard to imagine a world without these chemicals, for sure.
A quick glance of the table, which I’ve ordered from top to bottom in terms of caloric density, would suggest eating olive oil would be more “fattening” than eating starch since it contains more calories per gram, assuming you subscribe to Current Dogma.
But that same logic would also suggest eating coal would be more fattening than starch and gunpowder less fattening than ethanol. Gasoline would be more fattening than jet fuel. Hmmmm. Anyone interested in testing this hypothesis (personally)? Despite my wildest self-experiments, this is one self-experiment I’ll pass on. Why? Well for the same reason you’d pass on it – you know that there are far more important consequences to drinking diesel or snorting gunpowder than their relative energy densities.
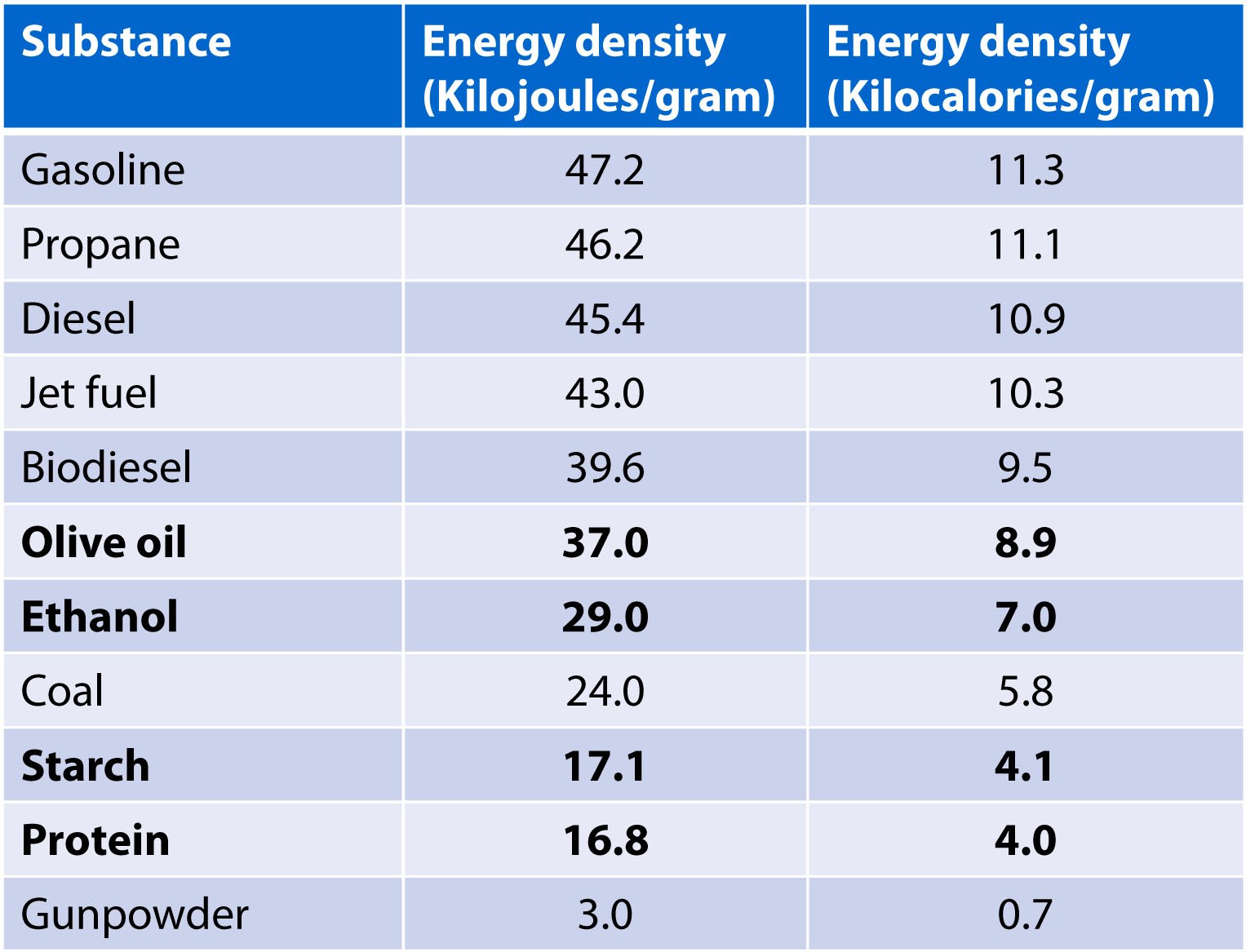
Sure, everything on this list is an organic molecule largely composed of the following four atoms: carbon, hydrogen, oxygen, and nitrogen. Not to bore everyone with a lesson on organic chemistry, but it’s the actual bonds between these atoms that are responsible for their energy densities. When you “liberate” (i.e., break) the bond between an atom of carbon and hydrogen, for example, you release an enormous amount of stored chemical energy. This table tells you exactly how much energy you would release if you were to break the bonds in these molecules, but that’s all it tells you. You can’t actually know, just by looking at this table, if jet fuel is more paraffinic than diesel or if gasoline has more isomerization than propane. And, you certainly have no idea, from the information contained in this table, of exactly how each of these substances will impact the hormones, enzymes, and cell membranes in your body if you ingest them.
Is it relevant to our bodies that olive oil has about the same energy density (i.e., calories) as biodiesel (also known as fatty-acid methyl-ester)? Or, is it more relevant to us that consuming olive oil has a very different effect on our bodies than consuming biodiesel beyond anything to do with the calories contained within them? Obviously consuming equal caloric amounts of olive oil versus biodiesel will have a very different impact on our body. Why then is it so hard to appreciate or accept that equal caloric values of olive oil and rice could also have very different impacts on our body?
The upshot
Let’s get back to the question you actually want to know the answer to. Do calories “matter”, and should you be counting them?
Energy density (calories) of food does matter, for sure, but what matters much more is what that food does in and to our bodies. Will the calories we consume create an environment in our bodies where we want to consume more energy than we expend? Will the calories we consume create an environment in which our bodies prefer to store excess nutrients as fat rather than mobilize fat? These are the choices we make every time we put something in our mouth.
Our bodies are complex and dynamic systems with more feedback loops than even the most elaborate Tianhe-1A computer. This means that two people can eat the exact same things and do the exact same amount of exercise and yet store different amounts of fat. Does it mean they have violated the First Law of Thermodynamics? Of course not.
Similarly, genetically identical twins can eat different macronutrient diets (i.e., differing amounts of fat, protein, carbohydrates) of the same number of calories, while doing a constant amount of exercise, and accumulate different amounts of fat. Does this violate the First Law of Thermodynamics? Nope.
What you eat (along with other factors, like your genetic makeup, of course) impacts how your body partitions and stores fat. In case anyone is wondering how I got over 2,000 words into this post without mentioning the i-word, wonder no longer. Insulin, while not the only factor involved in this process, is probably at the top of the list. When you eat foods that have the double whammy of increasing insulin levels AND increasing your cell’s resistance to insulin, your body prioritizes fat storage over fat utilization. No one disputes that insulin is the most singularly important hormone for causing fat cells to accumulate fat. Somehow the dispute centers on what causes people (full of billions of fat cells) to accumulate fat.
All calories are not created equally: The energy content of food (calories) matters, but it is less important than the metabolic effect of food on our body.
Photo by Aaron Barnaby on Unsplash




Thanks for this article. It is interesting.
whoah this blog is excellent i really like reading your posts.