One of the questions I most often receive: Does being in ketosis automatically translate to fat loss?
For those too busy to read ahead, let me give you the punch line: No. For those who want to understand why, keep reading (hopefully this is still everyone). This topic is — surprise, surprise — very nuanced, and almost always bastardized when oversimplified, which I’m about to do, though hopefully less than most. Without oversimplifying, though, this will turn into a textbook of 1,000 pages.
From the ketosis series, or at least the first and second part, along with the video in this previous post, you should have taken away that ketosis is not some ‘magical state of mystery.’ It’s simply a state of physiology where our liver turns fatty acid (both ingested and stored) into ketones.
There seems to be great confusion around ‘nutritional’ ketosis (a term we use to distinguish ‘dietary-induced’ ketosis from the other 2 forms of ketosis: starvation ketosis and ketoacidosis, the latter a serious complication of type I diabetes). But, before I try to dispel any of the confusion, we need to go through a little primer on what I like to call “fat flux.”
One point before diving in, please do not assume because I’m writing this post that I think adiposity (the technical term for relative amount of fat in the body) is the most important thing to worry about. On the contrary, I think the metabolic state of the cell is far more important. While there is a correlation between high adiposity (excessive fat) and metabolic dysfunction, that correlation is far from perfect, and, as I’ve discussed elsewhere, I think the arrow of causation goes from metabolic dysfunction to adiposity, not the reverse. But, everyone wants to lose fat, it seems, so let’s at least get the facts straight.
Let’s start with an assertion: Barring the presence of scientific evidence I’m unaware of, and barring surgical intervention (e.g., liposuction), reducing the adiposity of a person is achieved by reducing the adiposity of individual adipose cells, collectively. In other words, the number of adipocytes (fat cells) we have as an adult does not change nearly as much as their size and fat content. So, for people to reduce their fat mass, their fat cells must collectively lose fat mass.
Fat flux 101
According to “An Etymological Dictionary of Modern English,” the word flux comes from the Latin word fluxus and fluere, which mean “flow” and “to flow,” respectively. While the term has a clear mathematical meaning in physics, defined by a dot product I promise I won’t speak of, you can think of flux as the net throughput which takes into account positive and negative accumulation.
If we start with a bucket of water and put a hole in the bottom, the result, needless to say, is an efflux of water, or negative water flux. Conversely, if we start with a bucket – no hole – and we pour water in, that’s an influx of water, or positive water flux.
If that makes sense, then the idea of fat flux is pretty straight forward. If more fat enters a fat cell (called an adipocyte) than leaves it, the fat cell is experiencing a net influx – i.e., positive fat flux. And, if more fat leaves a fat cell than enters, the reverse is true: it is experiencing a net efflux, or negative fat flux.
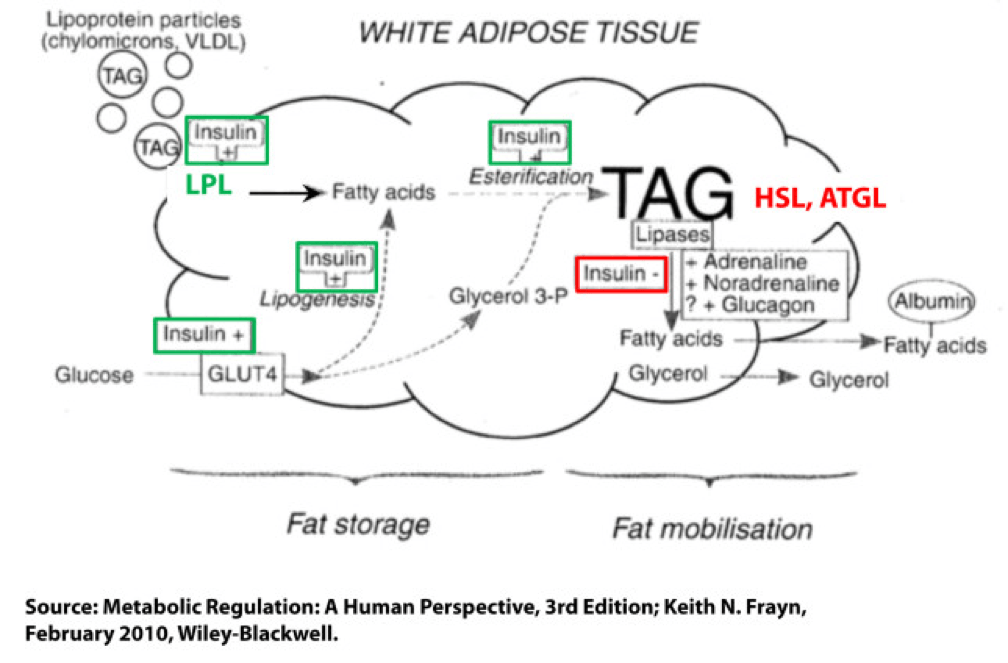
Not surprisingly, a fat cell is more complicated than a bucket. Basically, though, there are two “inputs” and one “output.” The figure below shows this in some detail. (TAG stands for triacylglycerol, which is another word for triglyceride, which is the storage form of fat.) The first thing you may appreciate, especially since I’ve highlighted it, is the role insulin plays in regulating the process of fat flux. Insulin does the following:
- Upregulates lipoprotein lipase (LPL), an enzyme that breaks down TAG so they can be transported across cell membranes. Since TAG are too big to bring across cell membranes, they need to be “hydrolyzed” first into free fatty acids, then re-assembled (re-esterified) back into TAG.
- Translocates GLUT4 transporters to the plasma membrane from endosomes within the cell. In other words, insulin moves the GLUT4 transporter to the cell surface to bring glucose into the cell.
- Facilitates lipogenesis, that is, facilitates the conversion of glucose into acetyl CoA which gets assembled into fatty acids along with glycerol.
- Facilitates esterification, that is, facilitates the process of assembling fatty acids into TAG (3 fatty acids per TAG).
- Inhibits hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL), two important enzymes that breaks down TAG into fatty acids and glycerol such that the fatty acids can be released from the fat cell. Once bound to albumin the free fatty acids are free to travel elsewhere in the body for use (e.g., to the liver for conversion to ketones, to the heart muscle or skeletal muscles for conversion to ATP).
- Though not shown in this figure, insulin appears to indirectly act on malonyl-CoA, a potent inhibitor of CPT I, one of the most important mitochondrial enzymes that facilitates the oxidation of fatty acids. (CPT I is what enables fatty acids to be shuttled into the mitochondria for oxidation, the process which releases or liberates their energy through electron transport.)
Other hormones and enzymes in the body also play a role. For example, under a sympathetic response, the so-called “fight or flight” response, adrenaline and noradrenaline (i.e., epinephrine and norepinephrine) activate HSL and ATGL to combat the effect of insulin as an inhibitor of lipolysis, thereby increasing lipolysis, or liberating stored energy from the fat cell. Glucagon may also play a role in this process, though the exact role is not as well understood, at least not in humans.
So in summary, insulin is indeed the master hormone that regulates the flow of fat (and glucose) into and out of a fat cell. There are other players in this game, to be sure, but insulin is The General. High levels of insulin promote fat storage and inhibit fat oxidation, and low levels of insulin promote fat mobilization or release along with fat oxidation.
If this sounds crazy – the notion that insulin plays such a crucial role in fat tissue — consider the following two clinical extremes: type 1 diabetes (T1D) and insulinoma. In the former, the immune system destroys beta-cells (the pancreatic cells that make insulin) – this is an extreme case of low insulin. In the case of the latter, a tumor of the beta-cell leads to hypersecretion of insulin – this is an extreme case of high insulin. Prior to the discovery of insulin as the only treatment, patients who developed T1D would become emaciated, if the other complications of glycosuria and dehydration didn’t harm them first. They literally lost all fat and muscle. Conversely, patients with insulinoma often present looking not just obese, but almost disfigured in their adiposity. Because Johns Hopkins is a high-volume referral center for pancreatic surgery, it was not uncommon to see patients with insulinoma when I was there. As quickly as we would remove these tumors, the patients would begin to return to their previous state and the adipose tissue would melt away.
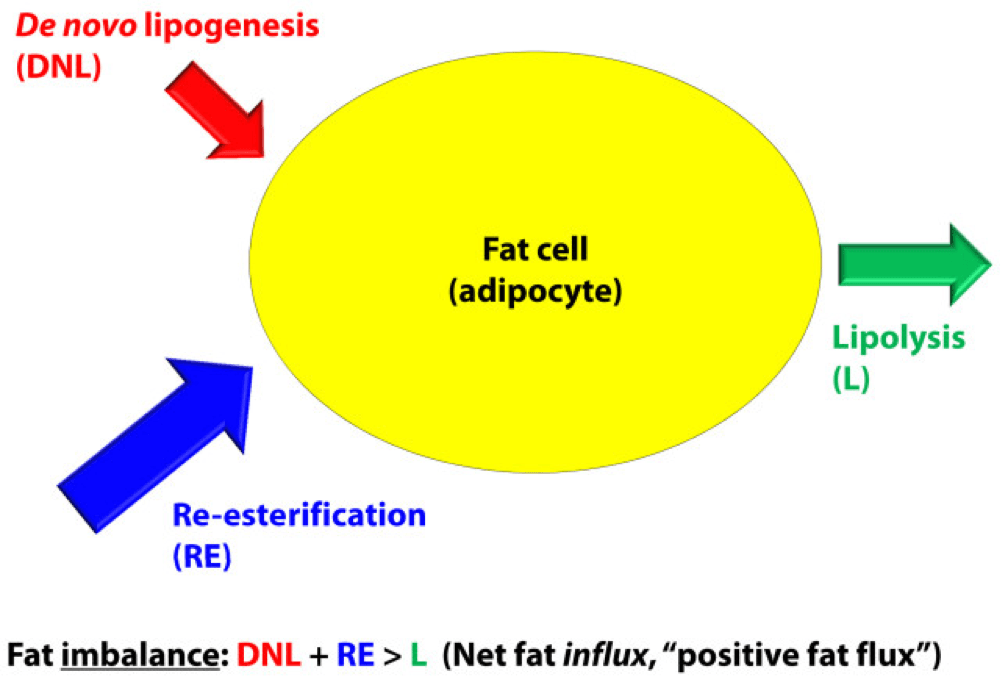
For the purpose of our discussion, I’ve simplified the more detailed figure above into this simplified figure, below. I’ve tried to size the arrows accordingly to match their relative contributions of each input and output.
The first figure, below, shows a state of fat balance, or zero net fat flux.
Input #1: De novo lipogenesis, or “DNL” – Until the early 1990’s there was no way to measure this directly, and so no one really had any idea how much this process (i.e., the conversion of glucose to fat) contributed to overall fat balance. Without going into great technical detail, , arguably one of the world’s foremost authorities on metabolomics and DNL, developed a tracer technique to directly measure this process. If I recall correctly, the original report was in 1991, but this paper is a great summary. Published in 1995 in the Journal of Clinical Investigation, this paper would go on to become the “citation classic.” This study demonstrated that under eucaloric feeding conditions, with about 50% of energy coming from CHO, DNL did not represent a significant contribution to fat flux. It was about 5%, hence the tiny red arrow under a state of fat balance (i.e., a state where fat entering the fat cell is equal to fat leaving the fat cell). A very important point to be mindful of, however, is this: this represents an average throughout the body and does not differentiate specifically between, say, DNL in the liver and DNL in the periphery (i.e., fat cells). This limitation is not trivial, but rather than focus on the very specific details of this paper, I’d rather use it as a framework for this discussion. (This paper is really interesting, and were it not for the fact that this post is going to be long enough, I would say much more about it. As such, I will probably do a full post on this paper and related topic in the future. The 1995 paper also examined what happened to DNL during periods of over- and under-feeding CHO and fat.)
Input #2: Re-esterification, or “RE” – In a state of fat balance, RE is largely composed of dietary fat sources that are not immediately used, but rather stored for later use. (Nuanced point: RE also includes fatty acids that were previously liberated from adipocytes, not oxidized, and are now being recycled back into TAG. This is a normal consequence of fat liberation. The fat cell probably ‘deliberately overdoes it’ by liberating more fatty acid from TAG just to be safe; that which is not oxidized is re-esterified. The exact balance of RE composed from dietary sources versus recycled fatty acids will depend on fat consumption and energy demands of the person. For the purpose of simplicity, this diagram does not show some portion of the L fraction returning to the RE fraction, though this is exactly what is happening in ‘real life.’)
Obviously, though, the relative size of the blue arrow depends on how much fat one is consuming and how many metabolic demands are in place for fatty acids. The latter is highly determined by dietary composition (see the discussion on RQ, or respiratory quotient, at about minute 31 in this video).
For the real aficionado, there is another wee bit of nuance here. This study, published in 1991 in the Journal of Lipid Research, suggested that the RE process is a bit more complicated than simply re-assembling fatty acids on a glycerol backbone inside an adipocyte. Based on these experiments, which used a similar* tracer method to the one used by Hellerstein et al. to evaluate DNL, the authors (which included Rudy Leibel, the co-discoverer of leptin) suggested that RE requires an intermediate step outside of the adipocyte in the interstitial and capillary space (figure 8 of the paper demonstrates this very well schematically).
(*) Technically, Hellerstein et al. used a heavy isotope; Leibel et al. used radioactive isotopes.
Output: Lipolysis, or “L” – Finally, in a state of fat balance, lipolysis must be equal to the sum of DNL and RE. This is true if we are talking about tiny little fat cells or giant ones. Remember, it’s the balance that matters.
I hope it’s clear from this summary that there are an infinite number of physiologic states that can satisfy the equation of fat balance: DNL + RE = L. For example, someone like me who is in fat balance (i.e., I’m neither gaining nor losing fat mass at this point) on a ketogenic diet with daily fat intake often exceeding 400 grams, has virtually zero DNL, but quite high RE, especially after meals. Consequently, I have very high L. If you took a person on a very low-fat diet (e.g., 20% fat, but 65% CHO), they would have modest DNL and low RE, but they would have low L. We would both be in fat balance, but we satisfy the equation DNL + RE = L by very different means.
OK, so let’s turn our attention to the non-equilibrium states: Net fat influx and net fat efflux.
Fat influx
In a state of net fat influx – accumulation of fat within a fat cell – the following condition must be met (on average): DNL + RE > L. (I say “on average” because, of course, a fat cell is a dynamic system with constant changes in these parameters. So, at any moment in time the balance can shift, but over a period of time the equation is correct.)
The next (overly simplistic) figure below gives you a representative state of what fat influx or ‘positive fat flux’ probably looks like. DNL is higher, but still relatively small, unless overfeeding CHO. RE is larger than it was in a balanced state, but not necessarily ‘huge.’ Most cases of net fat influx are probably governed by low L. In other words, fat accumulation is probably more governed by a failure to mobilize (breakdown TG into fatty acids for export and use) TAG than anything else.
Have you ever spoken with someone who is trying desperately to lose weight (fat) who says, “I don’t understand what’s happening…I hardly eat any fat, and yet I can’t lose a pound (of fat)!” The skinny people in the group scoff, right? Well, not so fast. It’s quite possible, if the hormones that regulate fat tissue are not working in your favor, to do such a poor job mobilizing fat from fat cells, and oxidizing that fat (see below), that you can be in fat balance, or even fat imbalance with accumulation, despite small DNL and small RE.
If you think about it, lipolysis (L), or liberating fat from a fat cell is a necessary, but not sufficient condition to actually generate the free energy inherent or stored within it. One more major step is necessary – oxidizing the fatty acid via the process of beta-oxidation. This is where one actually gets the energy (ATP) from fatty acids. The same hormones and enzymes that promote L, directly or indirectly act on other intermediaries that promote oxidation, more or less. The converse is also largely true.
Brief digression: I’m always troubled by folks who have never tried to take care of someone who is struggling to lose weight (fat), and who themselves have never been overweight, but who insist obesity is ‘simply’ an energy balance problem – people eat too many calories. When eternally lean people preach about the virtues of their ‘obvious’ solutions to obesity – just eat less and exercise more – I’m reminded of a quote (source unknown to me), “He was born on the finish line, so he thinks he won the race.” You only need to meet one woman with PCOS, or one person with hypothyroidism, or one child with Cushing’s disease to know that adiposity can – and is – largely regulated by hormones. The fact that such patients need to create a positive energy balance (i.e., eat more calories than they expend) to allow it does not seem to provide a meaningful insight into the mechanism of why.
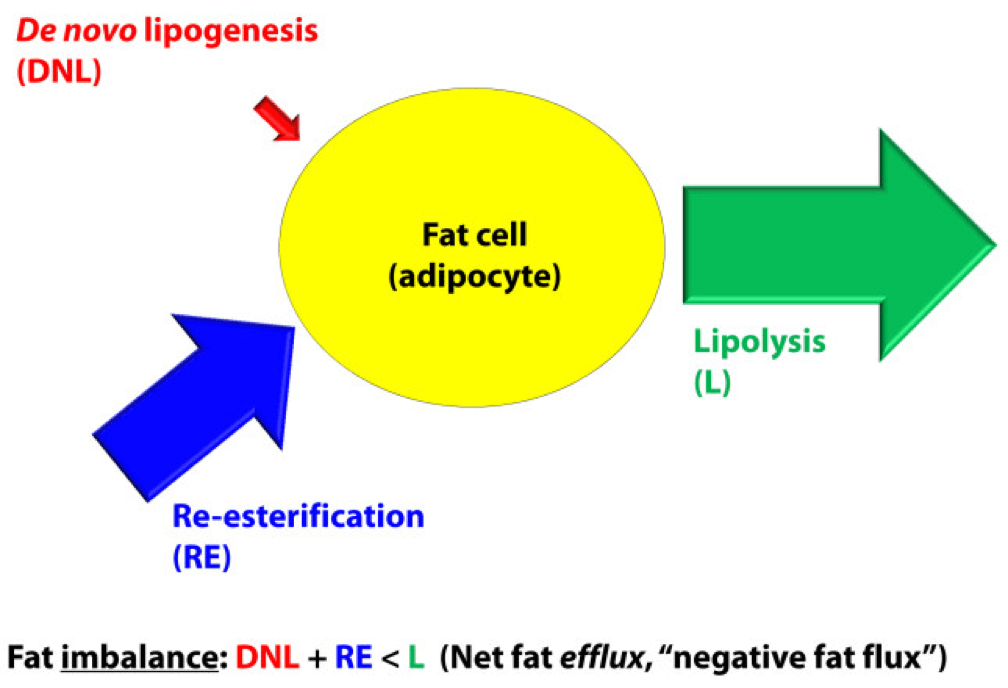
Fat efflux
In a state of net fat efflux – reduction of fat within a fat cell – the following condition must be met (on average): DNL + RE < L (same caveat as above on the idea of “on average”). Again, looking at the figure, you can see one physiologically common way this occurs, the setting of carbohydrate restriction. DNL is reduced (probably even to immeasurable levels, depending on the extent of restriction), but RE actually goes up. The net efflux, however, results from the greater increase in L.
A person in nutritional ketosis, if experiencing fat loss, probably looks like this. (Don’t worry, I have not forgot the opening questions: Does being in nutritional ketosis automatically put you in this state?). Certainly another state of net fat efflux is starvation. DNL and RE are both very small, especially DNL, and lipolysis is quite large. This is probably the most rapid state of negative fat flux a human can experience.
So what we do about it?
I do not believe there is only one state, shy of total starvation, which will assuredly put you in state of negative fat flux. Of course, starvation is not sustainable, and therefore should be taken off the table as a viable long term eating strategy.
What about profound caloric restriction? Yup, this is probably (though not necessarily) going to work, depending on how “profound” is defined. If defined as a 40% reduction of energy stable intake, it’s probably going to work. If defined as a 10% reduction, it would be difficult to know without knowing at least two other things:
- Baseline level of insulin resistance;
- RQ of pre- and post-diet.
What about dramatic alterations in macronutrient composition? This is where the discussion gets really interesting. Many people, myself included, advocate a diet that overall reduces insulin secretion. The rationale, of course, is provided by the first figure above (from the textbook) and a slew of clinical studies which I will not review here (see Gardner JAMA 2007, Ludwig JAMA 2012, and Shai NEJM 2008 to name a few).
But, the bigger question is why? Why do most (but not all, by the way) people with excess fat to spare who are on well-formulated carbohydrate-reduced diets lose fat? (Notice, I did not say weight, because the initial – and often rapid — weight loss achieved by many is actually water loss.)
Is it because of a physiologic change that leads them to reduce overall intake?
Is it because of a physiologic change that, despite the same intake in overall calories, increases their energy expenditure?
Is it some combination of these?
I wish I knew the answer, but I don’t (universally). I believe we will know the answer to this question in a few years, but until then, I’m left to offer the best my limited intuition can offer. In other words, what I suggest below is my best interpretation of the literature, my personal experience that I’ve had with hundreds of other people, and my discussions with some of the most thoughtful scientists in the world on this topic:
Thought #1: I suspect that many people who reduce simple carbohydrates and sugars end up eating fewer calories. This observation, however, may confound our understanding of why they lose weight. Do they lose weight because they eat less? Or, do they eat less because they are losing weight? I suspect the later. In this state, lipolysis — and by extension, given the hormonal milieu, oxidation — are very high, certainly relative to their previous state. By definition, L > DNL + RE, so there is ample ATP generated by oxidation of the fatty acid. If you believe (as I do*) that the liver is the master organ of appetite regulation, increases in ‘available energy’ (i.e., ATP) would naturally reduce appetite (though I don’t think we know if ATP per se is the driver of this feedback loop). But don’t confuse what’s happening. They are not giving up fat from their fat cells because they are eating less. They are eating less because they are giving up fat from their fat cells. Big difference.
(*) The especially astute reader will note that this is the first time I have made reference to this point. I have been heavily influenced recently by the work of Mark Friedman, and a discussion of this point is worth an entire post, which I promise to deliver at some point in the future. If you can’t wait, which I can understand, I highly encourage you to start scouring the literature for Mark’s work. It’s simply remarkable.
Thought #2: I also suspect that some fraction of people who follow this eating strategy lose fat without any appreciable reduction in their total caloric intake, at least initially. What?, you say, doesn’t this violate the First Law of Thermodynamics? Not at all. If L > DNL + RE, and the increase in lipolysis (i.e., fatty acid flux out of the fat cell) results in increased oxidation of fatty acids, energy expenditure (EE) would be expected to rise. A rise in EE, in the face of constant input, is a sign of fat loss. What differentiates those in this camp (I was in this camp) from those above (point #1), is unclear to me. It may have to do with concomitant exercise. I have seen unpublished data, which I can’t share, suggesting non-deliberate EE rises more in a low RQ (high fat, low carb) environment when a person is exercising significantly. I’m not stating the obvious – that the deliberate EE is higher – that is clearly true. I’m suggesting resting EE is for some reason more likely to rise in this setting. Since I’m taking the liberty of hypothesizing, I would guess this effect (if real) is a result of the body trying to keep up with a higher energy demand and making one trade-off (generating more free ATP via more lipolysis) for another (ensuring a constant supply of available energy to meet frequent demands). It is also possible that this increase in free/available energy results in an increase in deliberate EE (i.e., the person who suddenly, in the presence of a cleaned up diet feels the desire to walk up the stairs when they previously took the elevator). Finally, and perhaps most importantly, whether or not this up-regulation of energy takes place may be dependent on the other hormones in the body that also play a role in fat regulation, including cortisol, testosterone, and estrogen. They could be partly or mostly responsible for this. The literature is quite dilute with respect to this question, but in my experience (feel free to dismiss), it is not uncommon to see a reduction in cortisol and an increase in testosterone (I experienced about 50% in free and total testosterone) with a dietary shift that improves food quality. The same may be true of estrogen in women, by the way, though I have less clinical experience with estrogen.
Thought #3: As a subset to the point above (point #2), in an ‘extreme’ state of carbohydrate restriction, i.e., — nutritional ketosis — there is an energy cost of making the ketones from fatty acids. I referred to this as the “Hall Paradox” after Kevin Hall, who first alerted me to this, in this post (near the bottom of the post). What is not clear (to me, at least) is if this effect is transient and if so, how significant it is. I recall that during the first three months of my foray into nutritional ketosis, I was eating between 4,000 and 4,500 kcal/day for a 12-week period, yet my weight reduced from 176 lb (about 9.5% bf by DEXA) to 171 lb (about 7.5% bf by DEXA), which means that of the 5 pounds I lost in 12 weeks, 4 were fat tissue. Today, however, I don’t consume this much, closer to 3,800 kcal/day, and one reason may be that two years later my body is more efficient at making ketones and this so-called “metabolic advantage” is no longer present.
(I have always found the term “metabolic advantage” to be misleading, though I’m guilty of using it periodically. It’s really a metabolic disadvantage if your body requires more energy to do the same work, but nevertheless, people refer to – and argue vehemently about – this phenomenon. The question is not, does it exist? One look at individual summary data from David Ludwig’s JAMA paper on this topic makes that clear. The questions are, why does it only exist in some people, what relevance does it have to fat loss – is it cause or effect? – and, for how long does it persist?)
Thought #4: For reasons I have yet to fully understand, some people can only lose fat on a diet that restricts fat (and by extension a diet that is still high in carbohydrate, since I’m excluding starvation and profound caloric restriction from this discussion). In my experience (and Gardner’s A TO Z trial seems to validate this, at least in pre-menopausal women), about 20% of people aspiring to reduce adiposity seem to do it better in a higher RQ environment. Using the Ornish diet as the example from this paper, I suspect the reason is multifactorial. For example, the Ornish diet restricts many things, besides fat. It restricts sugar, flour, and processed carbohydrates. Much of the carbohydrate in this diet is very low in glycemic index and comes primarily from vegetables. So, I don’t really know how likely it is to lose weight on a eucaloric diet that is 60% CHO and 20% fat, if the quality of the carbohydrates is very poor (e.g., cookies, potato chips). The big confounder in these observations is that most low-fat diets, though still modestly high in RQ relative to a low-carb diet, reduce greatly the glycemic index and glycemic load, as well as the fructose.
Which brings us to the point…
Does being in nutritional ketosis ensure negative fat flux (i.e., fat loss, or L > DNL + RE)?
Being in ketosis tells us nothing about this equation! Let me repeat this: It is metaphysically impossible to infer from a measurement of B-OHB in the blood if this equation is being satisfied. It just tells us that our body is using some fraction of our dietary fat and stored fat (once it undergoes lipolysis) to make ketones, given that glucose intake is very low and protein intake is modest (net effect = minimal insulin secretion).
If you look at the figure below, you see this point (It’s simplified, obviously, and for example, does not show that fat from fat cells can be used directly by skeletal muscles). Nothing in this figure implies a reduction in the size of the cells at the bottom right of the figure. It’s quite possible, of course, since ketosis results in a large L and implies a very small DNL. But, if (small) DNL + (very large) RE is greater than (large) L, guess what? Fat flux is net positive. Fat is gained, not lost. Still in ketosis, by the way (quantified loosely by fasting levels of B-OHB greater than about 0.5 to 1 mM), but not losing fat. (I hope the first attempt at a solution in this setting is obvious by now, notwithstanding the fact that I’ve seen this situation dozens of times with more than one solution, including the ‘obvious’ one — reducing fat intake.)
The other myth worth addressing is that the higher the level of B-OHB, the more “fat burning” that is going on. This is not necessarily true at all. As you can tell, I love equations, so consider this one:
B-OHB (measured in blood) = B-OHB produced (from dietary fat) plus B-OHB produced (from lipolysis of TAG) less B-OHB consumed by working muscles, heart, brain.
How does knowing one of these numbers (B-OHB measured in blood) give definitive answers to another (B-OHB produced from lipolysis of TAG)? It can’t. That’s the problem with multivariate algebra (and physiology).
Many people who enter nutritional ketosis do so, I worry, because they believe it “guarantees” fat loss. I hope I have convinced you that this is not true. Nutritional ketosis is one eating strategy to facilitate negative fat flux, and it works very well if done correctly. It comes with some advantages and some disadvantages, just like other eating strategies. When I get back to the series on ketosis, I will address these, but for now I felt it was very important to put things in perspective a bit. Furthermore, I am convinced that it is not the ideal eating strategy for everyone.
Delorean by Marci Maleski is licensed under CC by 2.0





Good article. Quick Q.
If B-OHB (measured in blood) = B-OHB produced (from dietary fat) plus B-OHB produced (from lipolysis of TAG) less B-OHB consumed by working muscles, heart, brain…
…then would B-OHB (measured in blood) actually measure low, giving a false negative, in extremely active people with high amount of B-OHB consumed by working muscles, heart, brain?
Absolutely, Ben. This is, at least in part, why B-OHB in serum falls precipitously at activity above threshold. Part of the fall is feedback from HGO, but part of it is simply an overuse-relative-to-production issue. You may note in this situation, a paradoxical rise in serum B-OHB post work-out (See my figures of every 2 hours checks in the metabolic chamber and note in particular the levels post both workouts; this was in the video in previous post).
Thanks for the wonderful post! Following up on Ben’s question about the B-OHB equation….
“If B-OHB (measured in blood) = B-OHB produced (from dietary fat) plus B-OHB produced (from lipolysis of TAG) less B-OHB consumed by working muscles, heart, brain…”
Does this imply that your best chance of correlating a blood measurement of B-OHB to TAG Lipolysis would be in a fasted state in the morning? That is, in an AM fasted state, B-OHB from dietary fat is low (b/c you haven’t consumed any food for ~10hours) and consumption of B-OHB by the muscles is relatively low (b/c you’ve been sleeping). Given that, would the right side of the B-OHB equation above be dominated by B-OHB generated via lipolysis?
Even if this is the case, I understand having a high L doesn’t allow one to know the net average influx or efflux in the cell.
Just wondering if I am understanding this correctly. Thanks again!!
It might, Greg, but I’m not sure it does so with enough fidelity to warrant “taking to the bank.” I’ve just seen so much variation and so many inter-dependencies, that I can’t really believe any one number is indicative, besides the number that matters — actual mass of fat.
I would guess that “efficiency” comes into it somewhere along the line too – a well adapted muscle / brain may get enough ketones into its cells at a lower concentration than a ketone newbie.
“It is also possible that this increase in free/available energy results in an increase in deliberate EE .”
That would be me. I had been on the Atkins diet for about 3 weeks when I passed a noisy gym and saw women lifting weights. I hated gyms, and have my entire life.
But this day as I walked by, I suddenly thought, “that music’s really good.” And I went in and got a brochure. When I came home with the brochure my husband asked me “Where is my wife & what have you done with her?” I laughed but the next time I went past there, I did go in for the free class because “it just looked like fun!” Then I went back. . .do you know, going to the gym can be really fun?
Interesting. I had always been active, so I don’t believe this applied to me, but I’ve heard many suggest what you are suggesting.
Peter, any thoughts as to why a keto diet doen’t work for everyone? I think I read elsewhere that it doen’t work for up to 20% of people. Is there any research as to what diet will work for these people, or is there a “second best diet”out there that has the best chance of working for the keto-resistant folks?
Peter,
I hope you are planning on discussing at some point in the future why some people don’t respond the same
way when they go on a low carb diet. There are many people like me who have reached a plateau only after
losing a few pounds. Every time I read about somebody in nutritional ketosis who spontaneously start doing
intermittent fasting because they are just not hungry, I want to pull my hair out because I’m hungry. 🙁
Thanks.
That will imply I’ve figured it out.
Dr Steven Gundry talks about plateaus in his book “Diet Evolution: Turn off the genes that are killing you” – it’s all part of the normal process.
Hi Peter,
First of all, great read. I have some homework after you post new articles. Second, is it possible to really explain #6? The malonyl-CoA inhibition of CPT-1, and to a greater extent that whole pathway, is unbelievably important and deserves its own spotlight. I spent a few hours recently white-boarding it all out because not one of my 4 biochemistry textbooks puts that pathway into context. I had to hodgepodge together the difference between ketoacidosis and ketosis for a group of fellow students who look at the normal pathway and conclude that starvation and/or ketosis is some lesser form of ketocidosis. I’ve sat in rooms with doctors who talk patients out of ketosis because they know the pathophysiology more than the physiology. I’ve even had to suspend my knowledge when it comes to licensing examinations that lump the two together, or consider them gradations of the same disease.
And a favorite Grey’s Anatomy quote, “Eat when you can, sleep when you can, and don’t mess with the pancreas”
First things, first… That quote is actually wrong. It’s the TV version. The real quote, drilled into our heads during internship, was, “Eat when you can, sleep when you can, and never f*** with the pancreas!”
But, to your question, the 2 best papers I’ve seen on this topic are the following:
DIABETES, VOL. 51, JANUARY 2002 (Dysregulation of Fatty Acid Metabolism in the Etiology of Type 2 Diabetes)
Am J Clin Nutr 1998;67(suppl):500S–4S (Glucose–fatty acid interactions in health and disease)
Hi Peter,
Very thoughtful post, I like your style of ‘I’m not claiming I know all the answers, I’m trying to find out and explain what is actually going on’.
Anecdotally, I’ve been trying to gain weight while being, more or less, in ketosis during the last six months (I don’t measure ketone levels so I can’t say exactly how much of the time I’ve been in ketosis but I’ve been eating a very strict diet) only to realise that I’ve maybe gained 1kg (starting at around 51kg, 177cm). My point is that I’ve been eating around 3000kcal/day for an extended period without gaining weight, alluding to your point that there are so many different factors to factor in (pun intended). I’m not sure this is a positive thing either as I would probably be healthier at a higher weight.
Moreover, I also experienced how my appetite went away when I first started out dramatically reducing my carb intake. As you also mention, I believe this was due to the mobilisation of the fat I had stored. When I then dropped below 50kg I started feeling like crap and things got really bad. My point about this is that I think it was a combination of calorie restriction (simply eating ‘real food’ alone will work wonders for many in terms of appetite and weight loss, I believe) and a preference for burning fat.
I just wanted to chime in with some thoughts from the other side of why people usually come here. Keep up the good work Peter!
Best regards,
Hemming
Thanks, Hemming. In addition the available energy model of appetite regulation, malonyl CoA probably suppresses appetite, too.
So our liver is the Flux Capacitor – but Mr Fusion is what most people think our bodies are – eg picture the scene where Doc Brown is stuffing any old trash into it and says “We need fuel!”.
That, is calories in/calories out.
As an aside, Mr Fusion was surmised to run on cold fusion, which your mate Gary Taubes might have some things to say about… 🙂
Peter, thank you for another great post. Could you clarify your hormonal changes when you adopted your current diet? I thought I had read elsewhere that cortisol rises in a ketogenic diet. As a man I am of course interested in testosterone, so what you said about a 50 % rise sounded interesting. Don’t you think this results from ketosis per se or rather from a high fat / high quality / low carb diet? Much appreciated!
Insulin – lower
Cortisol – lower
Thyroid – lower
Testosterone – higher
These are my results, only, of course.
Maintaining adequately ‘low’ insulin levels seems to be 1 of the prerequisites to enter and maintain a state of nutritional ketosis.
1- Keeping this in mind, how big could someones DNL+RE get in a state of nutritional ketosis (say approximately 1mM) where their adipocytes are in net flax influx?
2- If this is in fact possible, could you think of a case study or example showing this despite adequately low insulin levels for this ketotic state?
Or maybe I’m missing something about individual insulin sensitivity…
Great post Peter!
Sorry, Raph, not sure I understand your question.
Peter, I know you said you don’t know much about how estrogen figures in, but as a middle-aged woman, I have a HUGE interest in it!
I read somewhere that fat cells either contained estrogen or were somehow linked to estrogen cells so that people with higher body fat had more estrogen. Have you ever heard anything about this and if so, can you point me in the direction of some references?
Judy, I’ll get to it in time.
Please, please, please address the issues that menopausal and post-menopausal women face in trying to lose weight when you can. I’m hoping you can offer some advice, because the traditional methods that work for men and work for younger women simply don’t work for us anymore.
When I hit menopause 8 years ago, I gained almost 50 pounds in 18 months with no changes in eating or activity (I’ve eaten low-carb since the late 1990s). Nothing would take off the pounds. Finally, I was able to take off 35 pounds using homeopathic hcg and kept it off for more than a year. Then, over the past year, I’ve gained back 20 pounds of it. It doesn’t come back gradually, but rather in spurts of about 5 pounds at a time. I’ll stick at one weight for months, and then all of a sudden have a spurt where suddenly I’m 5 pounds heavier, despite the fact that I’m still eating the same menus (typically 30 or fewer grams of carbs per day). I’m not gluten-free yet, but getting there. Also, I do take bio-identical hormones, and my gyno says the levels are good.
So what does a menopausal woman have to do to lose weight? I’m in “analysis paralysis” at the moment, not sure whether I should go higher in fat, higher in protein, lower in fat, etc. The very-high-fat approach (75-80% fat) doesn’t seem to work for me or for MANY if not most of the women I know in my age group. I know I feel the best when I’m eating around 100 grams of protein a day, but what of the other macros? I’ve tried the low-fat, higher-carb (coming from veggies and a little starch) approach, but I’m so freakin’ hungry all the time I can only do that for a couple days before my body screams out for some good fat.
Sometimes I feel like I’m not giving my body enough food (I rarely go above 1500 calories, emphasis on fat and protein). There’s argument that if you don’t give your body enough calories/nutrients, you risk starvation mode and your body gets really good at slowing down metabolism, exactly what you DON’T want. It’s hard for me to eat more than that, though. The one thing I love about low-carb eating is that you have a good breakfast, and then you’re not hungry for hours and hours and you don’t even think about food. I hate having to eat every couple of hours.
OK, I’m rambling now. Suffice it to say, if you could sometime address what women in menopause need to do to lose body fat, you’d make a whole lotta women very happy. Thanks!
Judy..
Check out my post further down. I’m a premenopausal woman who suddenly started to gain weight when my gynecologist started giving me extra estrogen. While many women suffers from low progesteron, giving estrogen really disturbes the balance of estrogen and progesterone.
For more information read on the website: mialundin.com
https://jcem.endojournals.org/content/89/4/1739.long is about obese post menopausal women.
“Thus, diet plus exercise training, but not diet alone, is effective in reducing chronic inflammation in obese postmenopausal women. In addition, modification of chronic inflammation is associated with changes in local adipose tissue metabolism in response to diet and exercise.” Includes lipolysis rate measurement.
Dr M Harvie has done work on intermittent energy / carb restriction in obese female populations of all ages, https://www.ncbi.nlm.nih.gov/pubmed/23591120 is her latest. May be worth a try – 2 consecutive days a week on <1000 calories with restricted carbs.
Peter, I happened to watch your TED talk and then found your blog from there. I really can’t wait to hear what your research finds on women and how their hormones interact with insulin and such. I never had a “weight problem” until after I delivered my 4th child when I was a few months short of 39 years old. 4 years later, I still have not lost the 40 lbs, I gained in that pregnancy despite being on a 1200 cal “balanced” diet and working out consistently. I did have gestational diabetes with all 4 of my pregnancies, but I also had all 4 of my kids close together starting at 34 and ending before I was 39. I was able to lose the weight between each pregnancy, but that last one did my metabolism in. My OB/GYN just tells me it is hormones and perimenopause. I have changed the foods I eat and am still trying to keep it at 1200 cal and have lost 4 lbs in a week. The fact that I have actually LOST even a pound after that long doing everything “right” is pure bliss! Thank you for your research. Although it often takes me several days to read a blog entry because I’m not a math or science whiz, it has been so worth the effort to understand it.
I surfed onto your blog “by accident”. I got kicked off the Engine 2 Extra website (fat free vegan eating), because I asked too many questions and have “complex” medical problems. Basically, many of my family members drop dead of MI’s in their early 40’s and 50’s; those who never smoke have bypasses and stents in their early 60’s and 70’s. I also have C282Y homozygous hemochromatosis, which is not a concern. I got my ferritin down from almost 500 to 8 in one year. It’s easy to control. Bloodletting is a miraculous cure. I am 51 and post-menopausal. I exercise regularly – weights 4x/week and cardio 3x/week. My BMI is 25. I gained some weight, especially around my belly, on the fat free vegan diet, but all my lab numbers – fasting glucose, cholesterol, triglycerides, etc. are “great”. Of course they don’t do the “fancy” cholesterol tests in Canada (apo-B, etc.), which I probably need with my family history. I look healthy and young for my age on the outside (as did many of my now deceased family members), but I know that I am a ticking time bomb on the inside. At 50+, my time is running out. Do you think I should try a ketogenic diet? I am willing to experiment on myself. I feel I have nothing to lose.
They should do apoB in Canada. You should insist on it. (You can let your doctor know that Allan Sniderman at McGill is basically the guy who figured out what apoB does.) I have no idea if a KD will be best for you, of course.
A digression based on my selfish concerns: as a competitive endurance athlete, my aim through trying to reach ketosis has not been fat loss per say (although I have enjoyed this), but the ‘metabolic flexibility’ that you have aimed for. In short, I never want to need another gel shot again. How far can I stray from a strict ketosis-inducing diet before I ‘fall off the wagon’ and lose metabolic flexibility? Or, once I develop greater insulin sensitivity, will my body ‘remember’ this sensitivity once I re-introduce some complex carbs back into my diet?
Good question, Rob. I don’t think it’s binary, though (the ketosis part is binary, but I think the metabolic flexibility improves over a range of RQ, IS). At some point — different for everyone, I suspect — you may laps back into glycogen dependence.
One thing that I dimly recall from my 1970’s medical physiology and biochemistry was the concept of “futile cycles.” These are biochemical cycles that don’t produce anything, net, but do burn energy. It struck me that the lipolysis and re-esterification could be exactly this–if those arrows are both large, isn’t it inevitable that more energy will be burned than if they are both small? Of course I have no idea how large an effect this might be.
Intuitively I think we all have the idea that our metabolic load is mostly based on activity (obviously wrong but intuitively appealing.) Of my 2200 Kcal/day I’ll bet all but about 400 is “metabolic”: Na-K pumping, circulation, “futile cycles” to maintain body temp, tissue maintenance, digestion, etc. It has always seemed to me that the body could respond to a low calorie diet with minor shifts in temperature generation etc to compensate and that might explain why people can eat less and not lose weight. We are too complex for the calories in calories out model.
Saw your video on Ted-X. I really appreciate your heart as you recall the patients you saw. In fact I’m seriously considering an article for the Christian medical association called “Judge not” based on what I’m learning here, and what I’ve seen in Mexico where type II diabetes is rampant among the desperately poor. You are doing good work.
Jim, I guess it depends on how one defines futile. My free-living EE is about 3,800 kcal/day (by DLW), but my resting (i.e., laying down for 24 hours) EE is about 2,100 kcal/day (in a metabolic chamber). Most of that 2,100 is the energy I require to make ATP to move ions across gradients, contract/relax involuntary muscles, and thermoregulation. But you’re right, most of our energy is not expended during periods of deliberate activity. Thanks for feedback on talk, also.
Just found your website (thanks to KTAR and a spotlight they did on you). I’m forwarding it to my son (a 2nd year med student), who, like me, believes in science but understands that findings can be misinterpreted and misapplied and may be based on the wrong question.
Although I have always worried about my weight (although never overweight), I now have found myself in the unenviable position of gaining weight as you did. The first burst happened perimenopausally…understandable but disconcerting. The last burst has happened 5 years later since my synthroid (given prophylactically, not as a result of hypothyroidism) was dramatically reduced when a DEXA scan showed normal bone density to osteoporosis in less than 5 years. Took on a personal trainer (I’ve always been physically active) but continue to grow. The doc said he doesn’t think the change in dosage relates…I’m (of course) insisting that nothing else changed but I did increase exercise on top of my daily 4 mile (in less than one hour) walk, so I’m thinking something metabolically has changed.
I’m a firm believer in “average” does not mean “individual” and that I will somehow need to find what will work for *me*. No problems with BP, cholesterol, etc., no meds (other than synthroid), no health issues but this has me frustrated. I will take into account your findings and thank you for being willing to think outside the box.
You said it spot on, Denise. What works on average or works for me, is not nearly as important as what will work for you. I hope everyone reading this will take a “selfish” view for a moment and realize that the answer to that question is most important. Pending more nuanced research, self-experimentation within the boundaries of scientific evidence is a good place to start.
Hi Denise
This website, this woman, is changing lives with science. Check it out. You’ll be amazed by her generosity and incredible discoveries.
https://sugarfreegoodies.info/
Interesting read for sure… I am currently doing KD (along with my wife) and honestly, while I do appreciate the lack of lows (and am not a fan of the lack of highs) in terms of energy (I’m more “level’ than the peaks and valleys with my old diet… but I digree. I found that I had the most luck eating “clean” – low fat high protein with plenty of vegetables and low sugar, and just lifting like a madman in the gym. Made it to 183 lbs and while I’m only 192 now (5’10” – really need to get to 170-175)… it just seems like such a long journey.
In theory 4 you state:
“In my experience (and Gardner’s A TO Z trial seems to validate this, at least in post-menopausal women), about 20% of people aspiring to reduce adiposity seem to do it better in a higher RQ environment.”
The title of that A to Z trial is “Comparison of the Atkins, Zone, Ornish, and LEARN Diets for Change in Weight and Related Risk Factors Among Overweight Premenopausal Women”.
I just wanted clarification on if your theory applies to pre or post-menopausal.
The reason my interest is piqued in this particular theory has to do with my wife. She has always struggled with her weight and while I’m not privy to the exact diagnoses she’s been given over the years (there were several beginning prior to our marriage…), she does indeed have endometriosis which has required surgery in the past and a cyst has been removed from her ovary so your reference to PCOS caught my attention although I’m not aware of her having that exact diagnosis. Part of her treatment after surgery (15 years ago) was Lupron injections which were described as “chemically induced menopause” by her Dr. at the time. That went on for about a year but she has a needle phobia so it eventually gave way to traditional BC pills that she just takes consistently with maybe 3 breaks a year for a period. Long story short is I suspect her hormones are all out of whack and that’s playing a large role in her weight struggles.
Personally, I’ve had great success in ketosis. I dropped 30 pounds (170-140) in 3 months. I really didn’t think I had more than 10 lbs. to lose so I was amazed at the results. In particular with how much better I felt. My wife on the other hand didn’t really lose any significant weight over the course of a year, maybe 5 lbs. or so. We eat 80% calories from fat so we’re “all in”. She did experience the other benefits of reduced blood pressure, more energy, etc. so it wasn’t a complete bust but of course, we all would like the added benefit of carrying less fat. I have little doubt that nutritional ketosis is probably the healthiest way to eat but if something like the Ornish diet could work to shed excess fat, I’m sure she’d favor that approach at least in the short term.
I’ve always thought that her issue was more about the inability to “mobilize” stored fat than it was about being “too efficient” at storing it and this post bolsters that theory. I know I’ve been rambling for a while so I’ll get to a question! She’s only 40 and is still able to have periods but I have a feeling her hormones are more closely aligned with a post-menopausal woman. What hormones do you think play the biggest role in that situation and is there any particular testing we could ask her doctor for? Would it be obvious like testosterone/estrogen or something more obscure?
This is more of a comment but it would appear that you believe people with a problem on the L side of the equation may fit into a category that does better on the fat restricted (and by extension, higher carb) diets. Did I interpret that correctly? By reducing the RE compared to a low carb, high fat diet, you hopefully don’t introduce more DNL into the equation because L is still the bottleneck.
It seems like “mobilizing” HSL and ATGL would be key to getting someone with an L problem on the right track. Any thoughts around how to make that happen? Hormones? I’m sure if you knew the definitive answer to that, we wouldn’t be having this discussion!
Chris, good catch. The study was in premenopausal women. Typo corrected. I think your suspicion is correct in your wife’s case, though it would hard for me to speculate on exact causes further. Would certainly suggest docs look at full spectrum of IR, all glucocorticoids, plus sex hormones, thyroid completely, including antibodies.
I don’t think I can say who does better on a low fat vs. a low carb approach, though it does appear that ability to oxidize fat predicts who does better on low carb (high fat). The L side of the equation, I suspect, is where people get into trouble and where the net influx starts.
Very interesting post. I need to re-read it to further digest. Reading through the comments I noticed you talk about how a ketogenic diet has impacted your personal thyroid levels. I’m wondering, is there a way to combat lowered thyroid levels while staying on a ketogenic diet.
It may not be necessary, depending on if the reduction in thyroid hormone produces any clinical symptoms. If so, may require replacement, but this seems to be the exception, not the rule.
I have looked into this phenomenon of lower thyroid on low carb diets, and concluded that it probably not just not harmful, but actually beneficial. I should probably rewrite it more succinctly for my blog, but I did report on it here: https://paleohacks.com/questions/78343/is-lowered-t3-resulting-from-a-low-carb-diet-problematic.
-Amber
Sorry, the link in the above was meant to point to my answer, not my question: https://paleohacks.com/questions/78343/is-lowered-t3-resulting-from-a-low-carb-diet-problematic/78345#78345. -Am
Thank you for a very interesting and well written post, Peter. The graphics help to clarify the basic concepts.
And extra thanks for including the references, both in the post and in your responses to questions and comments.
Wow – not to trivialize your earlier posts but this is the good stuff as far as I’m concerned. I had already gleaned that a ketogenic diet wasn’t a magic bullet by itself, even though I’ve lost weight (fat) on it. But my post-menopausal wife has not and I’ve plateaued at around 12% bf (according to my bathroom scale). I get really hungry when I’ve tried to fast so either this is my set point or there’s more fine tuning to do. Not that I’m complaining but I haven’t eaten a processed carb or potato in months. What I’m understanding is that it really is (calories in) – (calories out) but the hormones make each of those terms anything but simple.
Thanks so much for parsing this out for us, I really appreciate the rigor and honesty.
Paul, calories in less calories out is always true, it just provides no meaningful insight. Glad this helps.
My apologies if this was covered in another post, but: In your three day stay described in your ihmc video, did all of the measured energies, calories, fluids and solids, inputted and outputted net out with your beginning and ending weight? ( I am assuming that errors were small enough that this could be determined, I have no idea.)
On those days I lost about 300 gm of mass, probably fat mass, given my intake and output and nitrogen balance. I did not get into this during the presentation.
Your diagrams and explanations continue to be clear. Thank you for taking the time.
If someone is in a net fat efflux (and losing weight, whether or not they are in ketosis ), so that free fatty acids are leaving the adipocyte, would you see a significant rise in triglycerides on their lipid profile? Looking at your first diagram, in this situation of efflux, there would be lots of FFAs entering the bloodstream, bound to albumin, would they reform TAGs in the blood (with the need to be carried by a lipoprotein)?
Great question. You may see it transiently, but you you may not. The changes in concentration are so timing-dependent, that it probably matters a lot when blood samples are being taken. I do recall one TG level I had during rapid fat loss that was very high, but otherwise I sort of steadily went down from >150 mg/dL to less the 30 mg/dL.
When I lost weight my TG also went up just as my albumin is borderline high. My doctor was of the same opinion as you regarding the TG and didn’t comment on the albumin. I’m not sure if it makes medical sense to have a positive correlation between TG and albumin – maybe someone can comment on this.
Great article Peter!! Really juices me up to go out and learn more!
I’m 48, male and overweight. I have hypothyroidism and hypogonadism (guess I won the lottery here, but am on medication to correct) and I tried nutritional ketosis for about a month and really did not shed any weight. I was one of those people who believe that just being in ketosis would make me lose weight.
I would like to confirm my takeaway from this post – I now have to get off my butt and conduct my n=1 experiments to figure out my solution to make L > DNL + RE ! Any suggestion for a place to start?
Thanks again and keep these great posts coming.
Start experimenting!
“They are not giving up fat from their fat cells because they are eating less. They are eating less because they are giving up fat from their fat cells.”
Looking at things from this perspective feels very refreshing to say the least and it really does explain a lot. When i first came across this idea reading a book on low carb diet by the swedish MD Andreas Eenfeldt it just blew my mind. Thank you Peter for another wonderfully insightful blog post and congratulations on the very touching TED talk you gave earlier. You truly are an inspiration!
Thank you, Martin. I hope, of course, you understand that I am not unique in having these ideas. I’m but one of many in a vocal minority.