One of the questions I most often receive: Does being in ketosis automatically translate to fat loss?
For those too busy to read ahead, let me give you the punch line: No. For those who want to understand why, keep reading (hopefully this is still everyone). This topic is — surprise, surprise — very nuanced, and almost always bastardized when oversimplified, which I’m about to do, though hopefully less than most. Without oversimplifying, though, this will turn into a textbook of 1,000 pages.
From the ketosis series, or at least the first and second part, along with the video in this previous post, you should have taken away that ketosis is not some ‘magical state of mystery.’ It’s simply a state of physiology where our liver turns fatty acid (both ingested and stored) into ketones.
There seems to be great confusion around ‘nutritional’ ketosis (a term we use to distinguish ‘dietary-induced’ ketosis from the other 2 forms of ketosis: starvation ketosis and ketoacidosis, the latter a serious complication of type I diabetes). But, before I try to dispel any of the confusion, we need to go through a little primer on what I like to call “fat flux.”
One point before diving in, please do not assume because I’m writing this post that I think adiposity (the technical term for relative amount of fat in the body) is the most important thing to worry about. On the contrary, I think the metabolic state of the cell is far more important. While there is a correlation between high adiposity (excessive fat) and metabolic dysfunction, that correlation is far from perfect, and, as I’ve discussed elsewhere, I think the arrow of causation goes from metabolic dysfunction to adiposity, not the reverse. But, everyone wants to lose fat, it seems, so let’s at least get the facts straight.
Let’s start with an assertion: Barring the presence of scientific evidence I’m unaware of, and barring surgical intervention (e.g., liposuction), reducing the adiposity of a person is achieved by reducing the adiposity of individual adipose cells, collectively. In other words, the number of adipocytes (fat cells) we have as an adult does not change nearly as much as their size and fat content. So, for people to reduce their fat mass, their fat cells must collectively lose fat mass.
Fat flux 101
According to “An Etymological Dictionary of Modern English,” the word flux comes from the Latin word fluxus and fluere, which mean “flow” and “to flow,” respectively. While the term has a clear mathematical meaning in physics, defined by a dot product I promise I won’t speak of, you can think of flux as the net throughput which takes into account positive and negative accumulation.
If we start with a bucket of water and put a hole in the bottom, the result, needless to say, is an efflux of water, or negative water flux. Conversely, if we start with a bucket – no hole – and we pour water in, that’s an influx of water, or positive water flux.
If that makes sense, then the idea of fat flux is pretty straight forward. If more fat enters a fat cell (called an adipocyte) than leaves it, the fat cell is experiencing a net influx – i.e., positive fat flux. And, if more fat leaves a fat cell than enters, the reverse is true: it is experiencing a net efflux, or negative fat flux.
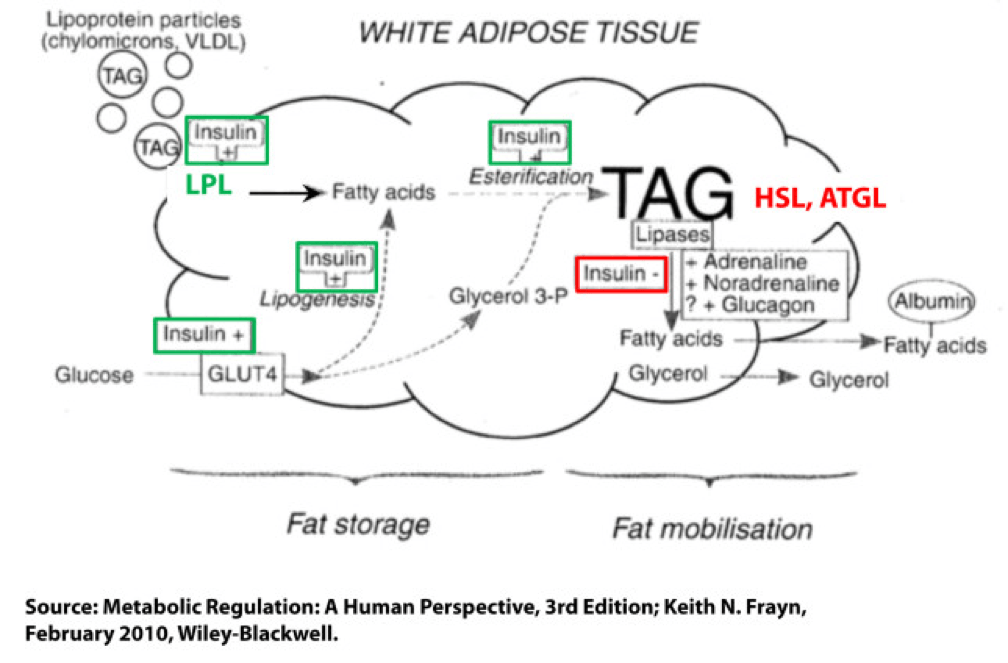
Not surprisingly, a fat cell is more complicated than a bucket. Basically, though, there are two “inputs” and one “output.” The figure below shows this in some detail. (TAG stands for triacylglycerol, which is another word for triglyceride, which is the storage form of fat.) The first thing you may appreciate, especially since I’ve highlighted it, is the role insulin plays in regulating the process of fat flux. Insulin does the following:
- Upregulates lipoprotein lipase (LPL), an enzyme that breaks down TAG so they can be transported across cell membranes. Since TAG are too big to bring across cell membranes, they need to be “hydrolyzed” first into free fatty acids, then re-assembled (re-esterified) back into TAG.
- Translocates GLUT4 transporters to the plasma membrane from endosomes within the cell. In other words, insulin moves the GLUT4 transporter to the cell surface to bring glucose into the cell.
- Facilitates lipogenesis, that is, facilitates the conversion of glucose into acetyl CoA which gets assembled into fatty acids along with glycerol.
- Facilitates esterification, that is, facilitates the process of assembling fatty acids into TAG (3 fatty acids per TAG).
- Inhibits hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL), two important enzymes that breaks down TAG into fatty acids and glycerol such that the fatty acids can be released from the fat cell. Once bound to albumin the free fatty acids are free to travel elsewhere in the body for use (e.g., to the liver for conversion to ketones, to the heart muscle or skeletal muscles for conversion to ATP).
- Though not shown in this figure, insulin appears to indirectly act on malonyl-CoA, a potent inhibitor of CPT I, one of the most important mitochondrial enzymes that facilitates the oxidation of fatty acids. (CPT I is what enables fatty acids to be shuttled into the mitochondria for oxidation, the process which releases or liberates their energy through electron transport.)
Other hormones and enzymes in the body also play a role. For example, under a sympathetic response, the so-called “fight or flight” response, adrenaline and noradrenaline (i.e., epinephrine and norepinephrine) activate HSL and ATGL to combat the effect of insulin as an inhibitor of lipolysis, thereby increasing lipolysis, or liberating stored energy from the fat cell. Glucagon may also play a role in this process, though the exact role is not as well understood, at least not in humans.
So in summary, insulin is indeed the master hormone that regulates the flow of fat (and glucose) into and out of a fat cell. There are other players in this game, to be sure, but insulin is The General. High levels of insulin promote fat storage and inhibit fat oxidation, and low levels of insulin promote fat mobilization or release along with fat oxidation.
If this sounds crazy – the notion that insulin plays such a crucial role in fat tissue — consider the following two clinical extremes: type 1 diabetes (T1D) and insulinoma. In the former, the immune system destroys beta-cells (the pancreatic cells that make insulin) – this is an extreme case of low insulin. In the case of the latter, a tumor of the beta-cell leads to hypersecretion of insulin – this is an extreme case of high insulin. Prior to the discovery of insulin as the only treatment, patients who developed T1D would become emaciated, if the other complications of glycosuria and dehydration didn’t harm them first. They literally lost all fat and muscle. Conversely, patients with insulinoma often present looking not just obese, but almost disfigured in their adiposity. Because Johns Hopkins is a high-volume referral center for pancreatic surgery, it was not uncommon to see patients with insulinoma when I was there. As quickly as we would remove these tumors, the patients would begin to return to their previous state and the adipose tissue would melt away.
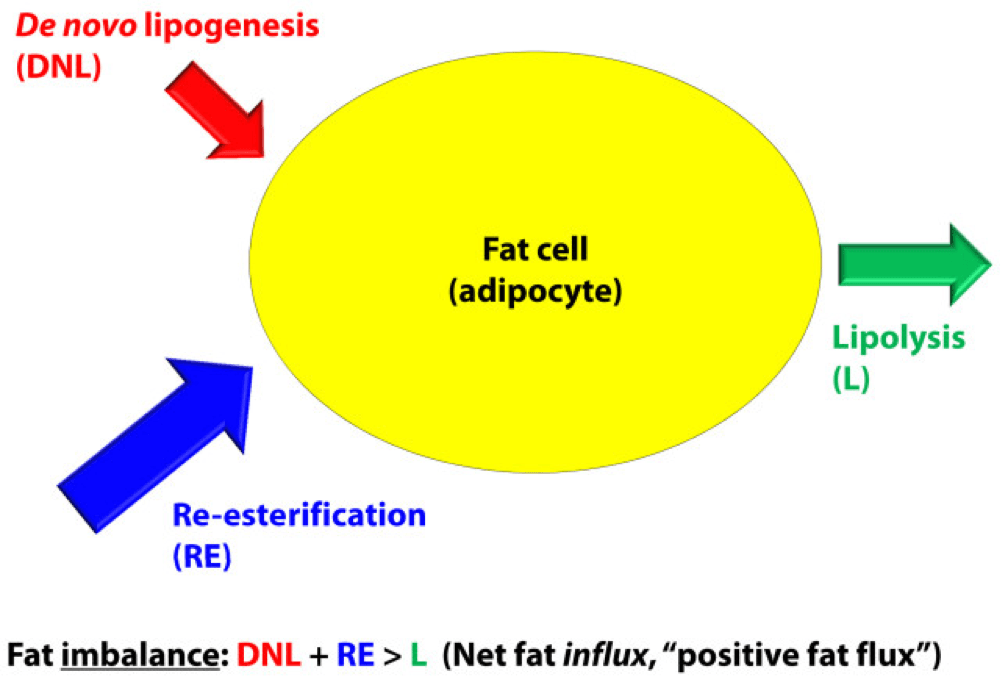
For the purpose of our discussion, I’ve simplified the more detailed figure above into this simplified figure, below. I’ve tried to size the arrows accordingly to match their relative contributions of each input and output.
The first figure, below, shows a state of fat balance, or zero net fat flux.
Input #1: De novo lipogenesis, or “DNL” – Until the early 1990’s there was no way to measure this directly, and so no one really had any idea how much this process (i.e., the conversion of glucose to fat) contributed to overall fat balance. Without going into great technical detail, , arguably one of the world’s foremost authorities on metabolomics and DNL, developed a tracer technique to directly measure this process. If I recall correctly, the original report was in 1991, but this paper is a great summary. Published in 1995 in the Journal of Clinical Investigation, this paper would go on to become the “citation classic.” This study demonstrated that under eucaloric feeding conditions, with about 50% of energy coming from CHO, DNL did not represent a significant contribution to fat flux. It was about 5%, hence the tiny red arrow under a state of fat balance (i.e., a state where fat entering the fat cell is equal to fat leaving the fat cell). A very important point to be mindful of, however, is this: this represents an average throughout the body and does not differentiate specifically between, say, DNL in the liver and DNL in the periphery (i.e., fat cells). This limitation is not trivial, but rather than focus on the very specific details of this paper, I’d rather use it as a framework for this discussion. (This paper is really interesting, and were it not for the fact that this post is going to be long enough, I would say much more about it. As such, I will probably do a full post on this paper and related topic in the future. The 1995 paper also examined what happened to DNL during periods of over- and under-feeding CHO and fat.)
Input #2: Re-esterification, or “RE” – In a state of fat balance, RE is largely composed of dietary fat sources that are not immediately used, but rather stored for later use. (Nuanced point: RE also includes fatty acids that were previously liberated from adipocytes, not oxidized, and are now being recycled back into TAG. This is a normal consequence of fat liberation. The fat cell probably ‘deliberately overdoes it’ by liberating more fatty acid from TAG just to be safe; that which is not oxidized is re-esterified. The exact balance of RE composed from dietary sources versus recycled fatty acids will depend on fat consumption and energy demands of the person. For the purpose of simplicity, this diagram does not show some portion of the L fraction returning to the RE fraction, though this is exactly what is happening in ‘real life.’)
Obviously, though, the relative size of the blue arrow depends on how much fat one is consuming and how many metabolic demands are in place for fatty acids. The latter is highly determined by dietary composition (see the discussion on RQ, or respiratory quotient, at about minute 31 in this video).
For the real aficionado, there is another wee bit of nuance here. This study, published in 1991 in the Journal of Lipid Research, suggested that the RE process is a bit more complicated than simply re-assembling fatty acids on a glycerol backbone inside an adipocyte. Based on these experiments, which used a similar* tracer method to the one used by Hellerstein et al. to evaluate DNL, the authors (which included Rudy Leibel, the co-discoverer of leptin) suggested that RE requires an intermediate step outside of the adipocyte in the interstitial and capillary space (figure 8 of the paper demonstrates this very well schematically).
(*) Technically, Hellerstein et al. used a heavy isotope; Leibel et al. used radioactive isotopes.
Output: Lipolysis, or “L” – Finally, in a state of fat balance, lipolysis must be equal to the sum of DNL and RE. This is true if we are talking about tiny little fat cells or giant ones. Remember, it’s the balance that matters.
I hope it’s clear from this summary that there are an infinite number of physiologic states that can satisfy the equation of fat balance: DNL + RE = L. For example, someone like me who is in fat balance (i.e., I’m neither gaining nor losing fat mass at this point) on a ketogenic diet with daily fat intake often exceeding 400 grams, has virtually zero DNL, but quite high RE, especially after meals. Consequently, I have very high L. If you took a person on a very low-fat diet (e.g., 20% fat, but 65% CHO), they would have modest DNL and low RE, but they would have low L. We would both be in fat balance, but we satisfy the equation DNL + RE = L by very different means.
OK, so let’s turn our attention to the non-equilibrium states: Net fat influx and net fat efflux.
Fat influx
In a state of net fat influx – accumulation of fat within a fat cell – the following condition must be met (on average): DNL + RE > L. (I say “on average” because, of course, a fat cell is a dynamic system with constant changes in these parameters. So, at any moment in time the balance can shift, but over a period of time the equation is correct.)
The next (overly simplistic) figure below gives you a representative state of what fat influx or ‘positive fat flux’ probably looks like. DNL is higher, but still relatively small, unless overfeeding CHO. RE is larger than it was in a balanced state, but not necessarily ‘huge.’ Most cases of net fat influx are probably governed by low L. In other words, fat accumulation is probably more governed by a failure to mobilize (breakdown TG into fatty acids for export and use) TAG than anything else.
Have you ever spoken with someone who is trying desperately to lose weight (fat) who says, “I don’t understand what’s happening…I hardly eat any fat, and yet I can’t lose a pound (of fat)!” The skinny people in the group scoff, right? Well, not so fast. It’s quite possible, if the hormones that regulate fat tissue are not working in your favor, to do such a poor job mobilizing fat from fat cells, and oxidizing that fat (see below), that you can be in fat balance, or even fat imbalance with accumulation, despite small DNL and small RE.
If you think about it, lipolysis (L), or liberating fat from a fat cell is a necessary, but not sufficient condition to actually generate the free energy inherent or stored within it. One more major step is necessary – oxidizing the fatty acid via the process of beta-oxidation. This is where one actually gets the energy (ATP) from fatty acids. The same hormones and enzymes that promote L, directly or indirectly act on other intermediaries that promote oxidation, more or less. The converse is also largely true.
Brief digression: I’m always troubled by folks who have never tried to take care of someone who is struggling to lose weight (fat), and who themselves have never been overweight, but who insist obesity is ‘simply’ an energy balance problem – people eat too many calories. When eternally lean people preach about the virtues of their ‘obvious’ solutions to obesity – just eat less and exercise more – I’m reminded of a quote (source unknown to me), “He was born on the finish line, so he thinks he won the race.” You only need to meet one woman with PCOS, or one person with hypothyroidism, or one child with Cushing’s disease to know that adiposity can – and is – largely regulated by hormones. The fact that such patients need to create a positive energy balance (i.e., eat more calories than they expend) to allow it does not seem to provide a meaningful insight into the mechanism of why.
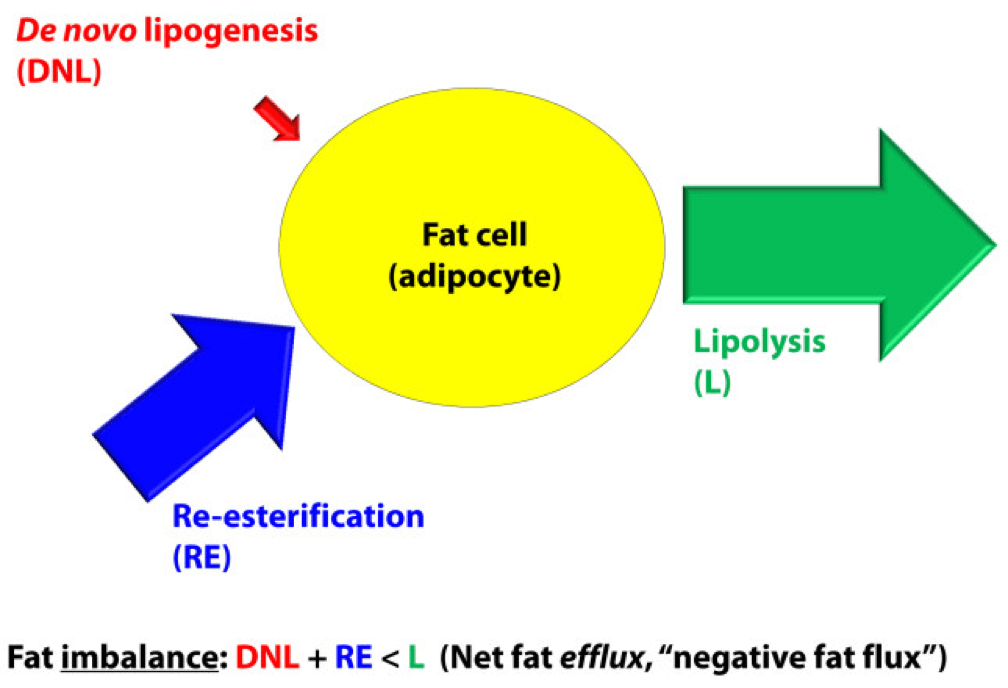
Fat efflux
In a state of net fat efflux – reduction of fat within a fat cell – the following condition must be met (on average): DNL + RE < L (same caveat as above on the idea of “on average”). Again, looking at the figure, you can see one physiologically common way this occurs, the setting of carbohydrate restriction. DNL is reduced (probably even to immeasurable levels, depending on the extent of restriction), but RE actually goes up. The net efflux, however, results from the greater increase in L.
A person in nutritional ketosis, if experiencing fat loss, probably looks like this. (Don’t worry, I have not forgot the opening questions: Does being in nutritional ketosis automatically put you in this state?). Certainly another state of net fat efflux is starvation. DNL and RE are both very small, especially DNL, and lipolysis is quite large. This is probably the most rapid state of negative fat flux a human can experience.
So what we do about it?
I do not believe there is only one state, shy of total starvation, which will assuredly put you in state of negative fat flux. Of course, starvation is not sustainable, and therefore should be taken off the table as a viable long term eating strategy.
What about profound caloric restriction? Yup, this is probably (though not necessarily) going to work, depending on how “profound” is defined. If defined as a 40% reduction of energy stable intake, it’s probably going to work. If defined as a 10% reduction, it would be difficult to know without knowing at least two other things:
- Baseline level of insulin resistance;
- RQ of pre- and post-diet.
What about dramatic alterations in macronutrient composition? This is where the discussion gets really interesting. Many people, myself included, advocate a diet that overall reduces insulin secretion. The rationale, of course, is provided by the first figure above (from the textbook) and a slew of clinical studies which I will not review here (see Gardner JAMA 2007, Ludwig JAMA 2012, and Shai NEJM 2008 to name a few).
But, the bigger question is why? Why do most (but not all, by the way) people with excess fat to spare who are on well-formulated carbohydrate-reduced diets lose fat? (Notice, I did not say weight, because the initial – and often rapid — weight loss achieved by many is actually water loss.)
Is it because of a physiologic change that leads them to reduce overall intake?
Is it because of a physiologic change that, despite the same intake in overall calories, increases their energy expenditure?
Is it some combination of these?
I wish I knew the answer, but I don’t (universally). I believe we will know the answer to this question in a few years, but until then, I’m left to offer the best my limited intuition can offer. In other words, what I suggest below is my best interpretation of the literature, my personal experience that I’ve had with hundreds of other people, and my discussions with some of the most thoughtful scientists in the world on this topic:
Thought #1: I suspect that many people who reduce simple carbohydrates and sugars end up eating fewer calories. This observation, however, may confound our understanding of why they lose weight. Do they lose weight because they eat less? Or, do they eat less because they are losing weight? I suspect the later. In this state, lipolysis — and by extension, given the hormonal milieu, oxidation — are very high, certainly relative to their previous state. By definition, L > DNL + RE, so there is ample ATP generated by oxidation of the fatty acid. If you believe (as I do*) that the liver is the master organ of appetite regulation, increases in ‘available energy’ (i.e., ATP) would naturally reduce appetite (though I don’t think we know if ATP per se is the driver of this feedback loop). But don’t confuse what’s happening. They are not giving up fat from their fat cells because they are eating less. They are eating less because they are giving up fat from their fat cells. Big difference.
(*) The especially astute reader will note that this is the first time I have made reference to this point. I have been heavily influenced recently by the work of Mark Friedman, and a discussion of this point is worth an entire post, which I promise to deliver at some point in the future. If you can’t wait, which I can understand, I highly encourage you to start scouring the literature for Mark’s work. It’s simply remarkable.
Thought #2: I also suspect that some fraction of people who follow this eating strategy lose fat without any appreciable reduction in their total caloric intake, at least initially. What?, you say, doesn’t this violate the First Law of Thermodynamics? Not at all. If L > DNL + RE, and the increase in lipolysis (i.e., fatty acid flux out of the fat cell) results in increased oxidation of fatty acids, energy expenditure (EE) would be expected to rise. A rise in EE, in the face of constant input, is a sign of fat loss. What differentiates those in this camp (I was in this camp) from those above (point #1), is unclear to me. It may have to do with concomitant exercise. I have seen unpublished data, which I can’t share, suggesting non-deliberate EE rises more in a low RQ (high fat, low carb) environment when a person is exercising significantly. I’m not stating the obvious – that the deliberate EE is higher – that is clearly true. I’m suggesting resting EE is for some reason more likely to rise in this setting. Since I’m taking the liberty of hypothesizing, I would guess this effect (if real) is a result of the body trying to keep up with a higher energy demand and making one trade-off (generating more free ATP via more lipolysis) for another (ensuring a constant supply of available energy to meet frequent demands). It is also possible that this increase in free/available energy results in an increase in deliberate EE (i.e., the person who suddenly, in the presence of a cleaned up diet feels the desire to walk up the stairs when they previously took the elevator). Finally, and perhaps most importantly, whether or not this up-regulation of energy takes place may be dependent on the other hormones in the body that also play a role in fat regulation, including cortisol, testosterone, and estrogen. They could be partly or mostly responsible for this. The literature is quite dilute with respect to this question, but in my experience (feel free to dismiss), it is not uncommon to see a reduction in cortisol and an increase in testosterone (I experienced about 50% in free and total testosterone) with a dietary shift that improves food quality. The same may be true of estrogen in women, by the way, though I have less clinical experience with estrogen.
Thought #3: As a subset to the point above (point #2), in an ‘extreme’ state of carbohydrate restriction, i.e., — nutritional ketosis — there is an energy cost of making the ketones from fatty acids. I referred to this as the “Hall Paradox” after Kevin Hall, who first alerted me to this, in this post (near the bottom of the post). What is not clear (to me, at least) is if this effect is transient and if so, how significant it is. I recall that during the first three months of my foray into nutritional ketosis, I was eating between 4,000 and 4,500 kcal/day for a 12-week period, yet my weight reduced from 176 lb (about 9.5% bf by DEXA) to 171 lb (about 7.5% bf by DEXA), which means that of the 5 pounds I lost in 12 weeks, 4 were fat tissue. Today, however, I don’t consume this much, closer to 3,800 kcal/day, and one reason may be that two years later my body is more efficient at making ketones and this so-called “metabolic advantage” is no longer present.
(I have always found the term “metabolic advantage” to be misleading, though I’m guilty of using it periodically. It’s really a metabolic disadvantage if your body requires more energy to do the same work, but nevertheless, people refer to – and argue vehemently about – this phenomenon. The question is not, does it exist? One look at individual summary data from David Ludwig’s JAMA paper on this topic makes that clear. The questions are, why does it only exist in some people, what relevance does it have to fat loss – is it cause or effect? – and, for how long does it persist?)
Thought #4: For reasons I have yet to fully understand, some people can only lose fat on a diet that restricts fat (and by extension a diet that is still high in carbohydrate, since I’m excluding starvation and profound caloric restriction from this discussion). In my experience (and Gardner’s A TO Z trial seems to validate this, at least in pre-menopausal women), about 20% of people aspiring to reduce adiposity seem to do it better in a higher RQ environment. Using the Ornish diet as the example from this paper, I suspect the reason is multifactorial. For example, the Ornish diet restricts many things, besides fat. It restricts sugar, flour, and processed carbohydrates. Much of the carbohydrate in this diet is very low in glycemic index and comes primarily from vegetables. So, I don’t really know how likely it is to lose weight on a eucaloric diet that is 60% CHO and 20% fat, if the quality of the carbohydrates is very poor (e.g., cookies, potato chips). The big confounder in these observations is that most low-fat diets, though still modestly high in RQ relative to a low-carb diet, reduce greatly the glycemic index and glycemic load, as well as the fructose.
Which brings us to the point…
Does being in nutritional ketosis ensure negative fat flux (i.e., fat loss, or L > DNL + RE)?
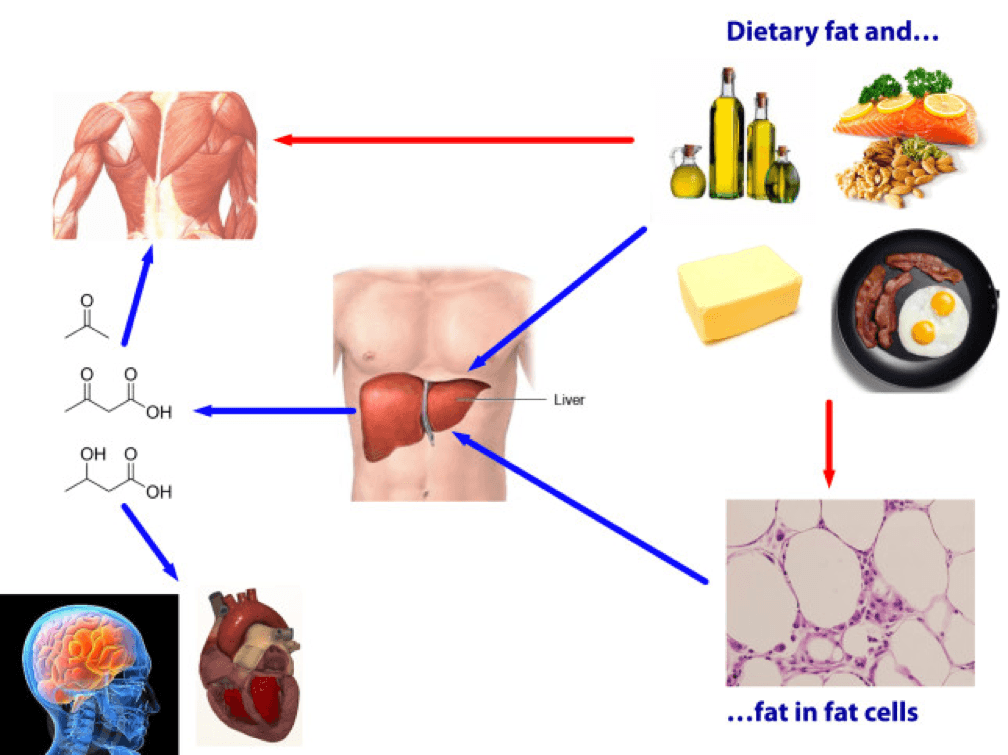
Being in ketosis tells us nothing about this equation! Let me repeat this: It is metaphysically impossible to infer from a measurement of B-OHB in the blood if this equation is being satisfied. It just tells us that our body is using some fraction of our dietary fat and stored fat (once it undergoes lipolysis) to make ketones, given that glucose intake is very low and protein intake is modest (net effect = minimal insulin secretion).
If you look at the figure below, you see this point (It’s simplified, obviously, and for example, does not show that fat from fat cells can be used directly by skeletal muscles). Nothing in this figure implies a reduction in the size of the cells at the bottom right of the figure. It’s quite possible, of course, since ketosis results in a large L and implies a very small DNL. But, if (small) DNL + (very large) RE is greater than (large) L, guess what? Fat flux is net positive. Fat is gained, not lost. Still in ketosis, by the way (quantified loosely by fasting levels of B-OHB greater than about 0.5 to 1 mM), but not losing fat. (I hope the first attempt at a solution in this setting is obvious by now, notwithstanding the fact that I’ve seen this situation dozens of times with more than one solution, including the ‘obvious’ one — reducing fat intake.)
The other myth worth addressing is that the higher the level of B-OHB, the more “fat burning” that is going on. This is not necessarily true at all. As you can tell, I love equations, so consider this one:
B-OHB (measured in blood) = B-OHB produced (from dietary fat) plus B-OHB produced (from lipolysis of TAG) less B-OHB consumed by working muscles, heart, brain.
How does knowing one of these numbers (B-OHB measured in blood) give definitive answers to another (B-OHB produced from lipolysis of TAG)? It can’t. That’s the problem with multivariate algebra (and physiology).
Many people who enter nutritional ketosis do so, I worry, because they believe it “guarantees” fat loss. I hope I have convinced you that this is not true. Nutritional ketosis is one eating strategy to facilitate negative fat flux, and it works very well if done correctly. It comes with some advantages and some disadvantages, just like other eating strategies. When I get back to the series on ketosis, I will address these, but for now I felt it was very important to put things in perspective a bit. Furthermore, I am convinced that it is not the ideal eating strategy for everyone.
Delorean by Marci Maleski is licensed under CC by 2.0





Thank you so much for another fine post on a fine blog. So glad to see Friedmans work being mentioned. His work was an eyeopener for me during my studies. Discussions pertaining to hunger and satiety often focus too much on the signals (leptin, ghrelin, PYY etc.) and too little on the source of the signal which seem to be highly related to ATP production. Anyway, keep up the good work!
I just realized an exception to this:
“How does knowing one of these numbers (B-OHB measured in blood) give definitive answers to another (B-OHB produced from lipolysis of TAG)? It can’t.”
When water fasting (supplemented, of course) the BHB in the blood is only the result of fat burning, as no fat is being consumed. Let’s say it’s after 3 days of water fasting, if it truly takes 3 days for food to clear the body as noted in allergen testing.
True, but not the case I was referring to. I’m addressing nutritional ketosis, not starvation ketosis.
To go even further in limiting this, it’s only when the bhb level is 2-3, somewhere between 5 and 10 days of fasting, that it is a useful measurement of how much is being burned. Above that, the bhb level may rise to 6 and above, but that only reflects lipolysis and how many ketones are available for the body to use and not actual use. Only those original 2-3 mmol of ketones are being used, the rest is present just in case and is not excreted or taken up by the brain, muscles etc. And then insulin kicks in again to limit ketone production to something below 10, so lipolysis decreases. Presumably once glucose or protein intake starts again, those last 4+ mmol of ketones in the bloodstream are stored again. So the real benefit of water fasting would be from when bhb levels hits 2 until it exceeds 3 as after that metabolism drops in response. After that nutritional ketosis is called for to keep metabolic rate up. (If I made a mistake somewhere there, please point it out as it would be very useful.)
Would make a neat esoteric article explaining: Why is nutritional ketosis superior to starvation ketosis for weight loss?
Great photo! That’s that flux capacitor from Back To the Future! 😀 did you like that movie doc? it’s my favorite
Hi Peter,
In a low carb/keto diet setting, cutting dietary fat sounds counter intuitive if one is following their hunger because if their bodies are releasing fatty acids accordingly, dietary fat would be adjusted via hunger signals unless they are force feeding themselves which makes me think that it could be something other than insulin that is inhibiting release of body fat for oxidation? So cutting fat might lead to hunger or short term weight loss at the cost of lower energy expenditure?
For those who have stalled, could it be that their energy expenditure has decreased (due to weight loss or maybe thyroid) and now their bodies are not tapping into their stored fat as before? Can eating even more help in this scenario by increasing energy expenditure? Or may be adding more carbs if they directly affect thyroid?
Hard to say because there are many mechanisms at play. T4 conversion to rT3 may be partly responsible in weight-reduced folks.
Dear Peter,
First of all, thank you for the amazing resource you have created here, which I have been digging into for the past couple of months with great enthusiasm. While the books of Phinney and Volek gave me a good start into the practicalities of NK, the Q&A material on your site has filled in a multitude of details. To find such a wealth of high quality information is wonderful enough, but coming as it does from someone whose priorities are to do with using good science to help further the wellbeing of others – that’s something very special indeed. All power to you and Gary Taubes for what you are setting out to do with NuSI especially.
I do have a couple of questions relating to your discussion above with Hemming, about the need for dietary fat to support ketosis. I have been taking regular blood BHB readings and that has been helping me get a feel for how different eating and training habits impact my level of ketosis. I’m surprised to see that (so far) nothing raises BHB levels more than total fasting. Going without food throughout a highly active rest day recently, I hit a morning BHB level of 5.7mM the following morning (at which point I hadn’t eaten for 32 hours). A couple of hours after that I ate what I thought was a highly ketogenic breakfast of 110g raspberries 100g heavy and clotted cream plus a couple of fish oil capsules. Two-and-a-half hours later, my BHB level had dropped to 3.8mM. Ok, there were 7-8g of CHO in the raspberries, but I still expected my BHB levels to stay up around where they were first thing. On a more normal day I might see a waking BHB level of around 1.2-1.6mM, do a fastest Crossfit session then eat lunch, after which my afternoon readings will often drop below 1.0mM for a considerable period. I’ve been careful over protein intake but the combined effect of a high-intensity workout and eating anything at all seem to combine to knock me out of NK on almost a daily basis. Plus, if my BHB (and presumably also therefore FFA mobilisation) levels are highest when fasting, and I wish to be leaner (currently around 13% BF, aiming for 8-10%), then it seems counterproductive to consume much dietary fat, as per Hemming’s comments above. I’m not talking about running some huge energy deficit that might slow metabolism, but the notion of consuming 2000kcal/day in fat when there are plenty of stores available and being mobilised seems illogical. Any thoughts or suggestions?
My second question leads on from the first, but is actually more fundamental, and while I feel slightly silly asking it, I looked long and hard for an answer here and via every other research medium I could muster, but still have come up blank. My question is, what is the underlying purpose of being in nutritional ketosis? Is ketosis just a proxy for low insulin level? Or, do you believe that by maintaining high serum BHB levels (particularly during training efforts of a high enough intensity that they would mostly rely on glycogen) we can encourage adaptations away from CHO metabolism towards use of a combination of ketones and FFA? Or, is there yet another aspect to the reasoning that I’ve missed? My observation from the meter is that really high-intensity workouts (particularly those involving sprint/interval structures with rest periods between) result in a massive blood glucose rises – from 4.9 to 11.8mM (i.e., 86 to a whopping 212mg/dl) on one occasion a couple of weeks ago – and this when fasted. Clearly as much as I would like to be spending the whole week in NK, my liver has other ideas when I am triggering major catecholamine releases with heavy Crossfit sessions. Research has confirmed that the BG rise is mostly from glycogenolysis rather than GNG (which makes sense, as GNG is a relatively slow process) but nothing in the literature that I’ve found has mentioned what happens to insulin levels in this scenario. It would be seem a safe guess that they’re also elevated, and that this causes a great deal of the circulating glucose to be converted back to muscle glycogen. This is a very anti-ketogenic environment and I find myself wondering whether I shouldn’t just “go with the flow” and introduce some dietary CHO and additional protein around workouts, given that I’m very likely being pushed out of NK anyway.
(Sorry, this post has turned out much longer than I intended. That’s the trouble with complex and nuanced subjects I guess!)
Warm regards,
Mike (London, UK)
Two really superb questions Mike,
I look forwards to Peter’ answers. it is interesting to note that the more that one goes down this road, the more one has such questions.
Excellent article.
I’m still trying to fully decipher it, I must admit.
I’ve personally lost 47 pounds, about 20% of my overall weight, and I’ve done it over the past year without changing any of the food I eat. Instead, I restricted how much I ate in the form of cutting out one meal, all snacks, and any drink with sweetener or sugar in it. (I don’t eat breakfast, but I have two meals between 1-8pm)
In addition, I exercise 3-4 times a week.
For me, the answer has always been, burn more calories than you’re taking in. Maybe that sounds too simplified, but I’ve lived it for over a year.
This diet isn’t as restrictive as it sounds. In addition, I eat anything in sight every other Friday from the moment I wake up until I go to sleep (if I want to…I don’t always want to).
I have about another 7 pounds to lose, and I think I’m going to have to finally cut carbs down.
Hi – I’ve been on a LCD for about 2 months and have lost about 6lbs. My problem is that my body fat scale (WithThings) doesnt actually reflect a drop in body fat. I know these scales use water to help measure body fat against adipose tissue in the body. Does being in a Ketogenic State (Diet), throw off the calculation? My jeans fit better so I know my body composition is changing. I suppose that’s the best test for results.
Peter, so if fatty acids enter the fat tissue via RE and DNL where they are assembled into TAG and then broken down into fatty acids and released back into the blood stream via lipolysis to be either oxidized or recycled via RE, what might cause a person not to be able to oxidize fat assuming they restrict carbs and/or are ketotic? How is beta oxidation inhibited or encouraged?
As a follow-up, since ketones are a by-product of fat oxidization, is it possible to be ketotic if your fat is not oxidizing and is simply being recycled.
Thanks, Seth
On the question of sensitive populations… I’m of Amerindian descent and my husband is European. we seem to thrive on very different diets – though his love of carbs does impact his dental health. I’m wondering if being Indian –only a few generations out from traditional diet –and close to menopause is a kind of double whammy w typ American Diet. I’ve always framed my craving for fat as “bar food” – but in truth I’m a scratch cooker – if I make leg of lamb the bones are broken and used for stock. this article https://www.westonaprice.org/health-topics/guts-and-grease-the-diet-of-native-americans/ helped me in reframing “fat” (not in your detailed scientific way) but as full use of the animal mentality. I also got advice from a Chinese medicine practicioner that bone broth would ease the transition to menopause. And (n=1 & possible placebo effects) drinking bone broth seems to have really helped reduce hot flashes. so far my personal experience and your writings seem more or less in synch.
the point of confusion for me is that the Chinese medicine practicioner argued that some carbs are good for menopause transition. I wonder why that might be?
Peter,
Is it possible to accumulate fat without insulin (e.g. type 1 diabetes)?
Paul
Without ANY insulin, probably not, or at least not much, but late stage T1D is extreme. Before the discovery of insulin such patients died completely emaciated–no fat and no muscle.
Dr. Attia,
I have been doing the ketogenic diet for about 2 months now, after finding your videos on YouTube. I feel great, have more energy, fewer cravings, and have lost weight. The first month I lost 14 lbs. The next month, 6 lbs. Now I am stalled at about 1 lb a week. I work out 4 days a week doing resistance, total body workouts with bursts of cardio. I am 5’8″ and 40 lbs. from healthy weight range. I use ketostix and keto calculator to ensure I am in ketosis, and am almost always in the mauve=eggplant color range. I am very careful with the macros (<30 net carbs, 90-100 protein, and the rest natural fats for 70-80%. Calories about 1300-1700 on workout days. I do daily IF as well, with a 6-8 hour feeding window. I am wondering what you do with your clients when their weight loss slows on Ketogenic diet, and how long before you consider them in a "plateau" that could indicate the need for changes. I am happy to do more IFIK if it will help, or to reduce or add calories. I just do not want to assume that the constant nutritional ketosis state is no longer working for me. I know I am very insulin resistant and gain weight with the slightest reintroduction of carbs in the diet. Can you speak to when to make changes, such as a fat fast, twice a week intermittent fasting, or trying something other than Ketosis with the assumption that it may be an indication that there is no longer appropriate fat flux?
I meant to say that I am only losing about 1/2 a lb. a week the last couple of weeks, not 1 lb.
Anna, 1 pound of fat loss per week is excellent progress. According to the work of Kevin Hall, it can take 3 years to reach steady-state reduced weight (though most of the weight loss occurs in the first year). Keep up the good work.
Peter, I’m 59, 160 lbs, Late Onset Type 1.5 diabetic and very active. My A1c was getting worse, up to 7.5+, so 8 weeks ago I took the Keto plunge. My blood glucose numbers were great for the first 3 weeks but then insulin resistance skyrocketed in the last 2-3 weeks. I’m waking up with 200+ blood sugar and taking more insulin now than before I was eating 70% fats. So I don’t know what to do, really. I was reading about “free fatty acid overload’ that can be a insulin blocker and using Niacin with some fruits and vegtables- by Ricky Everett. Do you know anything about that? Also, if you any lo carb doc close to Middle Tennessee, I’ll go in a minute. Even travelling now would be OK. Thanks, Mark
Takes me weeks to sort this out with my patients, but “typical” pattern of success involves metformin, CHO restriction (but not necessarily KD), and maybe GLP-1 agonists. But this is where you need a great doc. Sorry I don’t know any out there.
Hi Peter,
Been a long time my friend.
I know I asked you this before, and you approved it, but just wanted to confirm it one more time – I want to translate most of this post into Hebrew, and post it on my blog, with full credit to you and a link to your website of course.
Thanks!
BTW, you have a pretty big fan base in our ketogenic community in Israel 🙂
Of course, Aviv.
Hey Peter
If you ever wondered (which makes no sense), this is how this post looks like in Hebrew…
https://meeverlapaleo.blogspot.com/2015/01/blog-post_17.html
Thanks again, and congratulations on your cute new baby boy!
Very cool.
Hi Peter,
as always yet another highly informative article. Thank you for your dedication.
But it confused me. I’ve recently (2 months ago) embrace ketogenic lifestyle and lost 6 kg excluding the 2,5 kg water weight. During that time I didn’t exercise except archery session for 4 hours once a week.
The evidence of fat loss is obvious. I’ve gone from belt hole number 2 to number 5 which is 8 cm loss in waist measurement.
Yet the body fat percentage measured by Omron Body Fat Monitor shows no change at 22%. I was sure it’s not muscle wasting because I don’t feel weaker or sluggish. Do you have any suggestion what constitute my weight lost?
Not sure how accurate that device is for measuring body fat.
Dr. Attia,
I’ve read several of your posts so I didn’t really know which one to post my question, but decided on this one.
I’m still a little confused with conservation of energy and the ketogenic diet. I understand that carbohydrate restriction will cause a decrease in the insulin secretion. Since insulin increases fatty acid synthesis in the liver and a decrease in TAG breakdown, less circulating insulin would be beneficial that along with less glucose will shift the body’s energy source to fat. But at the end of the day I’m still failing to see the difference in net energy to a high carb diet with that has the same caloric level. (i.e. If one requires 2500 calories and consumes 2400 calories there will be an energy debt that will be taken from fat/muscle/glycogen stores. Those 2400 calories can be any combination of macronutrients)
The only thing that I can think of is would the body excrete non-metabolized nutrients during a ketogenic diet which would lead to the energy equation needing to include “energy excreted” to the equation like the following: Energy consumed – Energy burned – Energy excreted= net Energy consumption. I guess that the fact that you can detect ketones in the urine and acetone on the breath would be evidence that some energy is being wasted via excretion but is that the only thing responsible for the advantageous fat loss ketogenic diets have over standard low-fat diets?
Thanks!
Dr. Attia,
My question is in regards to fat oxidation measured by grams per minute verses exercise intensity (%V02 max).
If you have such a measurement, what has been your experience? I think .60 grams per minute is the maximum for a person on a non-ketogenic diet.
Fat ox (or CHO ox) is measured in g/min.
What turns a carb from A cellular to B? Why is whole wheat bread or blended fruit considered A cellular even if your also eating the fiber?
Hi Peter,
I have a question that is slightly off topic for this blog post, but still relevant to fat efflux. If someone experiences chronic insulin spikes that hinder their ability to breakdown stored fat for energy, does that make them more likely to suffer from deficiencies in fat soluble vitamins?
I understand that vitamins D, E, K, and A are all stored in the liver and in adipose tissue. To access the stored fat-soluble vitamins, does the body have to release stored fat or can it access it through other means?
Funny story about how this question actually came about. This morning I was sitting in the sun writing my med school personal statement. The major topic of the essay is about my experience as a personal trainer using a ketogenic diet to help clients lose weight and alleviate themselves of chronic disease. I actually used a quote from one of your blog posts in the essay…thanks for that! But anyway, I realized that I was getting a little too much sun and thought about vitamin D production and storage…at the same time that I was writing about weight loss/fat efflux. Thought you’d enjoy the mental loop.
I also wanted to thank you for all of your hard work on this blog and work in nutrition field in general. This blog changed my perspective on health, nutrition, scientific methodology, and medicine. It also pointed me in the right direction to find research from great scientists, like Phinney and Volek. Finding your blog was definitely one of the initiating factors that lead to my decision to pursue a career in medicine.
Thanks Peter!
Dr. Attia,
Thank you for taking the time to put this information and your experiences online, it is extremely helpful to beginners like me. I do find myself confused by the “information blender” between high carb and low carb diets. There is a video on the high carb/no meat protein website that outlines how high fat diets increase insulin resistance by blocking the signaling pathway between insulin receptors and glucose transport vesicles in individual cells. Obviously, I’m trying to lower my triglycerides so this came across as a shock when doing research on a high fat diet. Keep up the good work you do!
P.S. – Here is the video, https://www.forksoverknives.com/fat-insulin-resistance-blood-sugar/
Ryan
A high fat diet probably does increase IR in the presence of (relatively) high carbohydrate. High insulin + high fat is a bad combo (it’s called, the “standard American diet,” of course).
Hi Ryan,
The video did not mention what would happen to glucose uptake and insulin when muscles are filled with glycogen and can’t take any more glucose. Also, glucose tolerence gives very little information without measuring insulin levels. Insulin resistance is very complex issue; it is both desirable and harmful depending on the context. With regards to fat in the blood, you will find this study ineteresting:
https://feinmantheother.com/2012/02/22/saturated-fat-on-your-plate-or-in-your-blood/
On the subject of the standard American diet, I wonder what exactly is a high fat, high carbohydrate diet?
It could be interpreted to mean a low protein diet. But Americans are near the top when it comes to per capita meat consumption – and also have a high consumption of cheese and other milk products – so I don’t think there’s a lack of protein, in the country, per se.
Certainly a lot of the packaged foods people eat throughout the day fit the bill for high fat, high carbohydrate. (Same thing goes for desserts.) Yet, interspersed throughout those junk-food excursions appear to be meals that make up for the protein scarcity of cookies, cake, and chips.
It’s a weird dietary pattern.
Hey Peter,
Maybe you can help me with my extreme difficulties losing weight on a ketosis /paleo /any diet really, and continued weight gain on very low calorie diets (800-1200-1400-1500-1600)
You mentioned you have seen the extreme problem if insulinomas. I don’t think I have one, as my hypoglycemia has been on going for as long as I can remember. Including hating fruit and sweets as a child. And a family history of hypoglycemia with no one ever developing diabetes. (excluding one who had a pancrectomy).
My fasting blood sugar is 55-75
My triglycerides are 34
My Albumin is 3.3
With high fasting insulin, excellent cholesterol and very low testosterone for a woman.
I maintained a bmi of 27 since I was 15 always on a diet. But recently ballooned to obesity in a very short time on propranolol, likely due to its clear contraindication to never be given to those with asthma or hypoglycemia. (prophylaxis tx for chronic migraines). After a year, I haven’t been able to lose any of this excessive gain.
My postprandial hypoglycemia typically occurs within 45 minutes of eating, this includes all levels of carbs 0-75g. I do feel more neuroglycopenic hypoglycemia when there are more then 20g carbs in anything consumed. Remarkably, my postprandial blood sugar rarely exceeds ~100 when check very frequently.
I have been actively asking doctors and Endocrinologists for help for the past 15 years and they will not treat my hypoglycemia problems, trouble losing weight or hyperinsulinemia. (and all of the dieticians they have sent me to have no idea how to manage my hypoglycemia, and none of their suggestions help me lose weight)
1) How can I lower my insulin levels that don’t seem to have any correlation to dietary intake?
2) Knowing insulin inhibits lipolysis. And certain hormones (growth hormone, catecholamines, glucagon, ACTH, corticosteroids, T3) aid in the stimulation of HSL with in adipose tissue…. Is there any hope in trying to increase HSL stimulation to overcome hyperinsulinemia? Or is it simply better (healthier) to reduce insulin?
3) Since I can’t explain it to my doctors, but is it odd, or is there a physiological reason I’m almost never hungry? Is there some reason for abnormal ghrelin/ leptin in my hyperinsulinemia hypoglycemia? Even in hypoglycemia, I only remedy it with food consciously because I have neuroglycopenic symptoms. Typically food makes me feel worse, so I am prone to avoid.
Thank you for any wisdom you can give on this atypical situation!