Josh Rabinowitz is a Professor of Chemistry and Integrative Genomics at Princeton University, where his research focuses on developing a quantitative, comprehensive understanding of cellular metabolism through the study of metabolites and their fluxes. In this episode, Josh focuses the discussion on three main topics: metabolomics, NAD (and its precursors), and cancer metabolism. The metabolomics discussion starts with a broad definition of metabolism, metabolites, and fluxomics before diving deep into glucose metabolism, lactate as a fuel, movement of lactate, and the regulation of these substrates. He then gives a detailed explanation of the electron transport chain and Krebs cycle and their implications with respect to both drugs and nutrition while also explaining how NAD is central to the process of energy generation. He then discusses the age-related decline in NAD and what current literature says about efforts to increase NAD through intravenous or oral supplementation with the precursors NMN and NR, including whether doing so provides any advantage to lifespan or healthspan. Finally, Josh ends the conversation talking about cancer metabolism and how one particular intersection between cancer metabolism and immunotherapy might provide a hopeful outlook on the future of cancer treatment.
Subscribe on: APPLE PODCASTS | RSS | GOOGLE | OVERCAST | STITCHER
We discuss:
- Josh’s background and unique path to becoming a research scientist at Princeton [3:30];
- What sparked Josh’s early interest in metabolism [11:15];
- Metabolomics 101: defining metabolites and how they are regulated [16:30];
- Fluxomics: Metabolism as a system in action [26:00];
- The Randle Hypothesis: glucose and fatty acids compete as substrates for oxidation [33:30];
- The important role of lactate as an alternate fuel [36:30];
- Fasting lactate levels as a potential early indicator of metabolic dysfunction [48:00];
- The beauty of the Krebs cycle and the role of NAD in energy production [53:15];
- How the drug metformin acts on complex I of the electron transport chain [1:05:00];
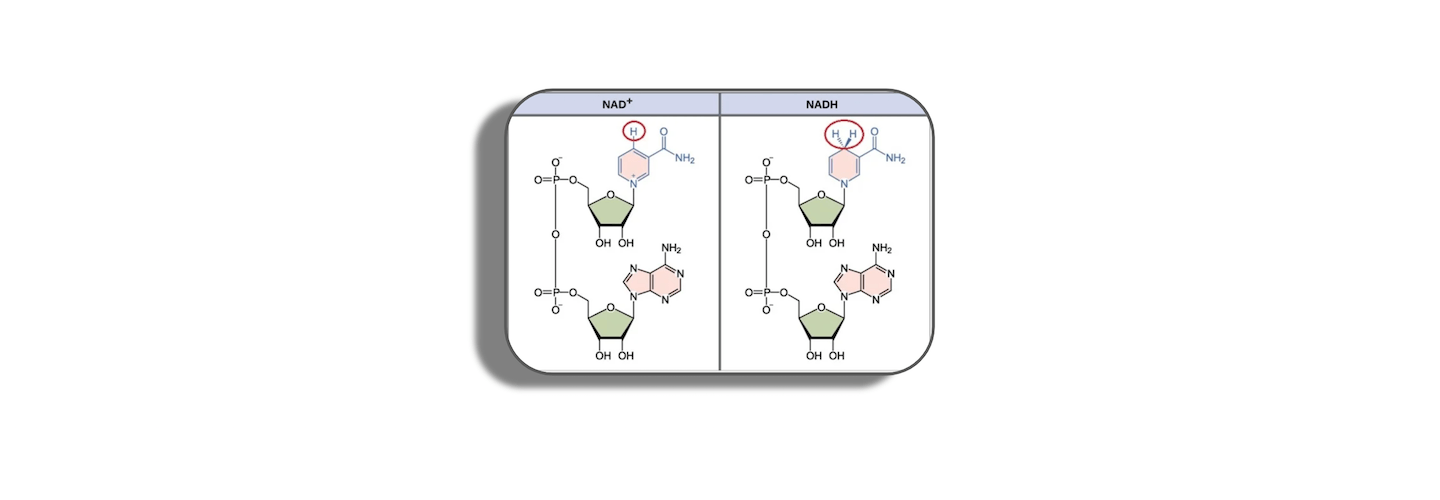
- The difference between NADH and NADPH [1:08:45];
- NAD levels with age, and the efficacy of supplementing with intravenous NAD [1:10:45];
- The usefulness of restoring NAD levels and efficacy of oral supplementation with NAD precursors NR and NMN [1:22:15];
- Exploring the hypothesis that boosting NAD levels is beneficial [1:32:30];
- Cancer metabolism and the intersection with immunotherapy [1:39:00];
- Making cancer a chronic disease: exploiting the metabolic quirks of cancer, augmenting the immune system, and more [1:46:15]
- The challenge of treating pancreatic cancer [1:50:30];
- Epithelial cancers that might respond to metabolic approaches to therapy [1:56:30];
- Josh’s hopeful outlook on the future of cancer treatment [1:59:00];
- Nutritional approaches to cancer attenuation [2:00:15];
- What makes Princeton University special [2:06:15];
- More.
Josh’s background and unique path to becoming a research scientist at Princeton [3:30]
- Peter recently interviewed 2 of their classmates from med school: Max Diehn and Karl Deisseroth
- He and Karl reminisced how they and Josh all started their surgical rotation together, in the same day almost 25 years ago
- Josh remembers Peter practicing a lot while he looked on
- He thought practice was what you were supposed to be doing if you wanted to become a surgeon
Josh’s PhD work
- Josh did his PhD with Harden McConnell (one of the great physical chemists) together with Mark Davis (the immunologist)
- His thesis was about the physical chemistry of T-cell activation
- At that time, people had discovered that different antigens could activate T-cells differentially
- He was really interested in how that happened; he studied the process of antigens turning into peptides that combined to MHC
- Then, how the kinetics of the interaction between the peptide antigen and the major histocompatibility complex protein, MHC, and then the interaction of that complex with the T-cell receptor determined whether people have productive or failed immune responses
- The hope was you could then manipulate those processes to promote better vaccination or disease clearance and also to potentially treat autoimmunity
Did Josh also have an interest in cancer? This work would be one of the hallmarks of how immunotherapy is effective in eradicating cancer.
- At that point in his career, he didn’t think immunological approaches to cancer held much promise
- He was more focused on infectious disease and autoimmunity
- He remarks, “Shows you what I know; it’s wonderful to see that the world has turned out to be a lot better than I dreamed it would be on that dimension”
- Peter adds, this is such an interesting topic; he had Steve Rosenberg on the podcast last year
- It was a beautiful and fun recap of how the immune system works
- #177 – Steven Rosenberg, M.D., Ph.D.: The development of cancer immunotherapy and its promise for treating advanced cancers
- The part of T cell activation that is amazing is the T cell receptor recognizes things that are 9-11 amino acids long
- The peptides have to be just be right size to be presented and then recognized
- This doesn’t seem to leave a lot of margin for error; it’s a really attuned system
Do you have a sense of why evolution ended up with such a narrow fragment of peptides that were recognizable, as opposed to a broader/ different range?
- Josh has an intuitive sense that our bodies work on the scale of billions of immune cells and billions of immune receptors that are made through recombination
- That naturally pairs with billions of antigens
- You just think about this number 9 and you think about the number of amino acids, right?
- There are 20 amino acids, so you’re talking about 20 to the 9th power of presentable peptide antigens
“These things are all tuned to be on the same scale, this scale of billions”‒ Josh Rabinowitz
- If the T cell recognized 3-amino acid peptides, that would not provide enough information to selectively respond to a virus or bacteria
- If it was 25 peptides, this would provide too much information
- Josh thinks the system was built to work on a minimal, or just the right amount of information
When in your training did Josh make the decision to be a full-time scientist, as opposed to a physician scientist?
- He applied for an internship knowing that he really loved research
- As he spent more time in medicine, he realized it involves a lot of doing the same thing over and over again
- There is a lot of following the standard of care
- His passion is to come in and try to do something different every day
- To think differently than people ever have before
- This is what led him to research
- He loved the patient interaction part of medicine, but doing things the same every day was challenging
- Peter agrees, “I think those of us that chose the more medical side of things can also speak to the frustration of how creativity can often be stifled in medicine”
- This is in large part, for good reason
- Surgery, probably more than other disciplines tends to frown upon creativity and novel approaches to problem solving
Before joining the faculty at Princeton, did you go straight into industry from medical school?
- Yes, Josh was fortunate enough to have the opportunity to work with one of the great early biotech entrepreneurs, Alex Zaffaroni
- They started Alexza Pharmaceuticals when he was straight out of medical school that was focused on fast drug delivery
- What could you do by being able to deliver medications noninvasively on the timescale of giving an IV Push in the hospital?
- They did that through inhalation of small molecules
- Building on the concept that if you smoke something like a cigarette, you get incredibly rapid access to the systemic circulation
- That was Josh’s first job
- The company’s initial focus was on migraines
- They never found the drug that had the perfect combination of safety with rapid delivery and efficacy
- They ultimately had a drug approved for acute agitation; it’s a sedative hypnotic
- Patients come in the ER, they’re agitated and frustrated and want to stop feeling that way
- Patients are eager to take a puff of something
- They calm down in a couple minutes; it’s wonderful for them
What sparked Josh’s early interest in metabolism [11:15]
What made Josh decide to take this lateral step to go into academics?
- He was lucky to go straight from that job into a faculty position at Princeton
- That’s a rare opportunity
What was the first problem he wanted to build his lab around?
{end of show notes preview}

Josh Rabinowitz, M.D., Ph.D.
Joshua Rabinowitz earned his BA in chemistry and BA in Mathematics at the University of North Carolina at Chapel Hill. He earned his PhD in Biophysics and MD at Stanford University. After graduating from Stanford University, he co-founded Alexza Pharmaceuticals and served as its Vice President for Research until 2004, when he joined Princeton University, where he is now a Professor in the Department of Chemistry & Lewis-Sigler Institute for Integrative Genomics and the Director of the Ludwig Princeton Branch. Since 2008, He has also been a Member of the Rutgers Cancer Institute of New Jersey.
Josh has received several awards for his scientific contributions— including the Allen Distinguished Investigator Award, the NIH Pioneer Award, the CAREER Award of the National Science Foundation and the Beckman Young Investigator Award. [Ludwig Cancer Research]





It would have been interesting to pursue the CD38 angle a bit more. I have seen the hypothesis that senescent cells secrete CD38, and that this may be cause of the decline in NAD with age.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4911708/
https://onlinelibrary.wiley.com/doi/10.1111/acel.13589
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3609577/
Two things: 1) George Brooks, UC Berkeley would be a great guest given the interest in lactate. I think he would provide an interesting perspective of lactate’s role in metabolism.
2) Love the acknowledgement of lactate, not pyruvate, as the endpoint of glycolysis and the formation of lactate as a balance between glycolytic flux and mitochondrial oxidation of pyruvate and NADH. Given that LDH is a near-equilibrium reaction and thinking of this as a hydrodynamic analogy where flux = water flow, mito density = pipe diameter: Considering that mammals can double their mitochondrial density with endurance training, then the amount of ‘head pressure’ i.e. [La] will be smaller in the trained vs untrained animal at the same flow (flux). Neither of which has anything to do with the amount of O2 available – in contrast to classic textbook description of the production of La and availability of O2 during exercise.
Practicing derm here. Love the podcast and content but I think Peter is missing it by not mentioning the derm literature on oral nicotinamide.
Dermatologists have been using nicotinamide supplements since the mid 1980’s to treat immunobullous disease and for much of the past decade for delaying onset of actinic keratosis and NMSC’s (which are very much an age related phenomenon). It seems at least somewhat relevant to the discussion.