If I held a crystal ball 10 years ago, I’m not sure I would’ve believed it if it showed me the increased interest in the ketogenic diet would look like the figure below. That’s 2 logs, folks.
Admittedly, I started my journey on this path in 2009, with a deep dive into ketosis in the Spring of 2011, but it seemed so obscure! (For a timeline of what I did, I think I covered it somewhere in this talk…yes I’m too lazy to actually confirm this by skimming through it.) All told I spent approximately 3 years in the strictest state of nutritional ketosis (NK) with one very memorable deviation when I had 6 or 7 full-sized and upsettingly decadent desserts circa September 2013. I believe the diet helped me transition from metabolic syndrome to metabolic health and I certainly thought it could benefit other people. This nutritional state could gain some steam, I thought.
I was well aware of the dearth of mainstream knowledge of NK, and particularly the conflation of NK with diabetic ketoacidosis (DKA), a pathologic state that results from the complete or near absence of insulin, which is what prompted my writing and desire to share my journey. And I was once in the wanker category of folks who spoke with “authority” about ketosis, despite knowing somewhere between zero and nothing on the topic. I remember exactly where I was sitting in a clinic at Johns Hopkins in 2002 during my residency explaining to (admonishing, really) a patient who was on the Atkins diet how harmful it was because of DKA. Not only that, the ketogenic diet could be seen as the antithesis of a “healthy” diet by conventional standards. I could see how this was a difficult proposition for many to acknowledge.
The beautiful part of good science is its self-correcting nature. The ugly part is this self-correcting nature often moves at a glacial pace—and it’s not linear. We often view history century-by-century and see what amounts to continual progress in medicine. But we live our lives—and consume information—day-by-day, exposed to the peaks and valleys of medical wisdom.
Looking back on my earlier posts on ketosis—and explaining what I eat, for example—makes me both chuckle and cringe. I remember how bizarre the diet seemed to many readers and the general public at the time. I also remember digging into the literature and learning, for example, that my alma mater, Johns Hopkins had been using the ketogenic diet to treat pediatric epilepsy for almost a century…and being so embarrassed about admonishing that patient I saw in my residency.
Since then, it’s safe to say I dove down the rabbit hole. The more I learned, the more I grew tired of reading so much misinformation on the topic. While there are more thoughtful people and articles on the subject of ketosis these days (e.g., here’s a thoughtful video on ketosis and ketogenic diets from one of my most important ketosis mentors, Steve Phinney, a co-founder of Virta Health1Disclosure: I’m an investor in, and advisor to, Virta Health.), there are still pieces like the one Vox published this month, that doesn’t exactly do the topic justice.
Like many variables in diet, health, and disease, it behooves us to look beyond the bumper sticker explanation. I want to highlight a couple of posts I wrote, to attempt to provide a little more nuance and understanding to the subject: “Ketosis — advantaged or misunderstood state?” Parts I and II. Part I follows below. I’m hoping to write more on the topic in the not-too-distant future since there’s been a number of intriguing papers published recently (certainly since 2012). But I also wanted to bring these back into focus in light of the information I’m seeing more of on the interwebz. (You can also visit the Ketosis section of the site to view more articles on the subject.)
Because I know people will ask, I have not been on a ketogenic diet “regularly” since about mid- to late-2014. The reasons are too nuanced to describe here, but my deviation is not because I lost confidence in its efficacy. With nearly a decade of clinical experience, I can safely say I was an outlier (in the best sense) with respect to my physiology and response. I was leaner, and more mentally and physically fit during this three year period than during any other period of time as an adult, and my biomarkers were as good as they had ever been. I’ve also seen the benefit of ketogenic diets first-hand on my patients and my own sister, a remarkable story I hope to share one day. But I’ve also been humbled by my inability to explain why some people have suboptimal or even negative responses to NK. I would say, all things considered, my knowledge of ketosis is greater today than when I was writing about it voraciously, but my confidence in my understanding of it, might actually be lower. As the saying goes, the further one goes from shore, the deeper the water gets.
—P.A., April 2018
§
(Part I: originally posted November 26, 2012)
In part I of this post I will see to it (assuming you read it) that you’ll know more about ketosis than just about anyone, including your doctor or the majority of “experts” out there writing about this topic.
Before we begin, a disclaimer in order: If you want to actually understand this topic, you must invest the time and mental energy to do so. You really have to get into the details. Obviously, I love the details and probably read 5 or 6 scientific papers every week on this topic (and others). I don’t expect the casual reader to want to do this, and I view it as my role to synthesize this information and present it to you. But this is not a bumper-sticker issue. I know it’s trendy to make blanket statements – ketosis is “unnatural,” for example, or ketosis is “superior” – but such statements mean nothing if you don’t understand the biochemistry and evolution of our species. So, let’s agree to let the unsubstantiated statements and bumper stickers reside in the world of political debates and opinion-based discussions. For this reason, I’ve deliberately broken this post down and only included this content (i.e., background) for Part I.
What is ketosis?
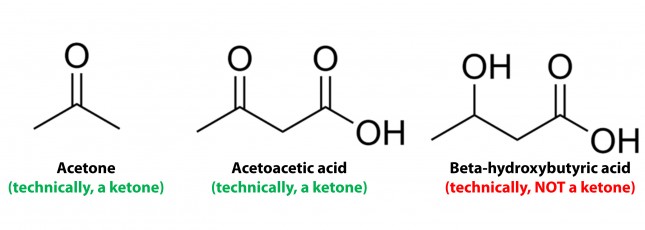
Ketosis is a metabolic state in which the liver produces small organic molecules called ketone bodies at “sufficient” levels, which I’ll expand upon later. First, let’s get the semantics correct. The first confusing thing about ketosis is that ketone bodies are not all – technically — ketones, whose structure is shown below. Technically, the term ketone denotes an organic molecule where a carbon atom, sandwiched between 2 other carbon atoms (denoted by R and R’), is double-bonded to an oxygen atom.
Conversely, the term “ketone bodies” refers to 3 very specific molecules: acetone, acetoacetone (or acetoacetic acid), and beta-hydroxybutyrate (or beta-hydroxybutyric acid), shown below, of which only 2 are technically ketones. (The reason beta-hydroxybutyrate, or B-OHB, is not technically a ketone is that the carbon double-bonded to the oxygen is bonded to an –OH group on one side, technically making B-OHB a carboxylic acid for anyone keeping score.)
Now, back to the real question at hand. Why would our body make these substances? To understand why or when the body would do this requires some understanding of how the body converts stored energy (the food we eat or the energy we store in our body, i.e., fat or glycogen) into phosphate donors. For a refresher on this process, please refer to the video in this post, specifically the section from 2:15 to 13:30.
The ATP issue
As you may recall, about 60% of the energy we expend, say 1,800 kcal/day for someone consuming 3,000 kcal/day in weight balance, is purely devoted to keeping us alive by generating enough ATP (“energy currency”) to do 2 things: allow ion gradients to function and allow muscular relaxation. So, obviously, we can’t tolerate – literally even for one minute – insufficient ATP production. In fact, one of the most potent toxins known to man (cyanide) exerts its effect on this process by inhibiting the electron transport chain which generates the bulk of the ATP our body produces. Even the most transient interruption of this process is fatal.
Take home message #1: No ATP, even for 1 minute, equals no life.
The brain issue
The brain is a particularly greedy organ when it comes to energy requirement. To put this comment in perspective consider the following: though our brain represents only about 2% of our body mass, it accounts for about 20% of our energy expenditure. (In children, by the way, this may be closer to 40-50% of basal metabolic demand.) So, beyond the ATP issue, above, there is a substrate issue with the brain as neurons derive most of their energy from glucose. While there is emerging evidence that neurons can also oxidize fatty acids directly in small amounts and may even prefer lactate (over glucose), these two substrates do not approach the levels of consumption by neurons that glucose does. So, for the purpose of this discussion, let’s just focus on the need of the body to provide glucose to the brain.
You’ll recall, from the point I made above, that my brain requires about 400 to 500 kcal of glucose per day (100 to 120 gm). You’ll also recall (from the video, above) that I can store about 100 to 120 gm of glucose in my liver. While I can store much more in my muscles, (on the order of about 300 to 350 gm), because muscles lack the enzyme glucose-6-phosphatase, glucose stored in muscle as glycogen is unable to re-enter the bloodstream and is meant for the muscle and the muscle alone to use. In other words, muscle glycogen is a stranded asset of glucose in the body to be used only by the muscle.
So, if I’m deprived of a dietary source of glucose, I depend solely on my liver to release glycogen (a process known as hepatic glucose output, or HGO). How long can HGO supply my brain with sufficient glucose? It depends on a few things that impact both the “source” and the “sink” of glucose. Other competing sinks for glucose (e.g., activity level, thermogenic needs) and sources (e.g., glycerol and gluconeogenic amino acid availability) can make a difference for a while. But, in a state of starvation we’ve only got about one to three days before we’re in trouble. If our brain doesn’t get a hold of something else, besides glucose, we will die quite unceremoniously.
Take home message #2: No glucose for 24-72 hours equals the need for something else the brain can use instead (that is not fat or protein, since neurons can’t oxidize fat and the last thing we want to do is start muscle wasting at a geometric rate).
The Krebs Cycle
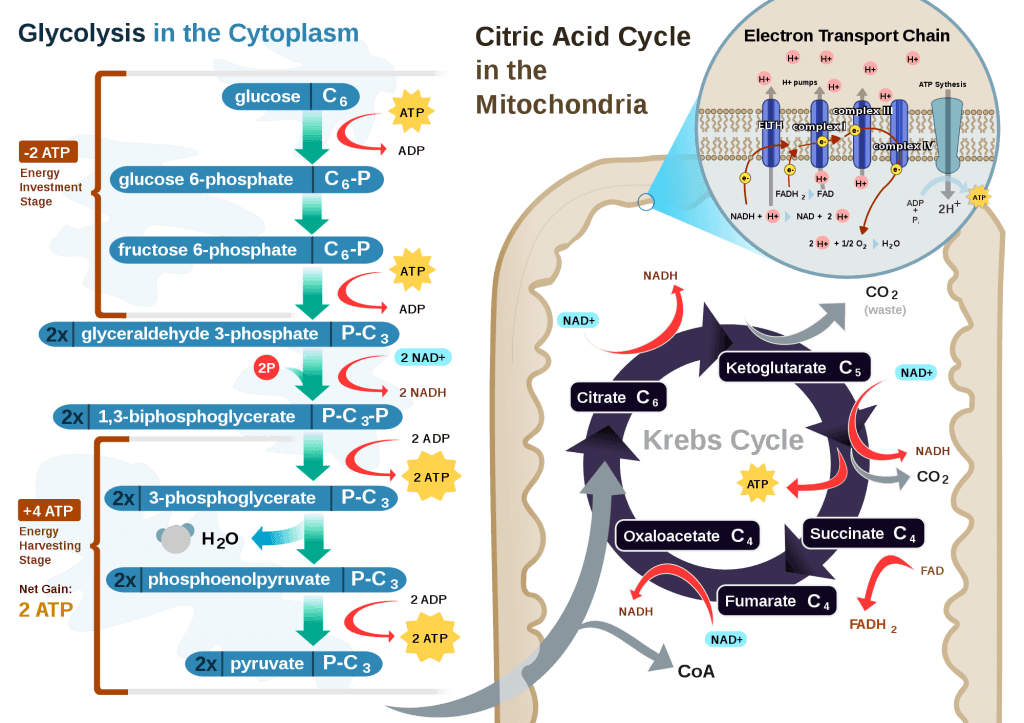
This poses a real evolutionary dilemma. We need an enormous amount of energy just to not die, but the single most important organ in our body (also quite energy hungry in its own right) can’t access the most abundant source of energy in our body (i.e., fat) and is, instead, almost solely dependent on the one macronutrient we can’t store beyond a trivial amount (i.e., glucose). Obviously our species wouldn’t be here today if this were the end of the story. But, to understand how we survived requires one more trip down biochemistry memory lane. In the figure below (also included and described in the video) I gloss over a pretty important detail.
How, exactly, does our body take pyruvate (from glucose) or acetyl CoA (from fat) and generate so much ATP? The answer lies in the beauty of the Krebs Cycle, which feeds into a process called the electron transport chain (or ETC), I alluded to above. Since the adage ‘you can’t get something for nothing’ is as true in biochemistry as it appears to be in life, to get all that ATP (i.e., stored energy in the form of the phosphate bond), we need to give up something. What the ETC does give up, as its name suggests, is electrons. Through a series of redox reactions the ETC trades the stored energy held by electrons going from higher to lower energy states in exchange for the chemical energy stored in the bonds of the third phosphate group on an ATP molecule.
To think of it another way, if you start with stored energy – glucose or fat, for example, which if burned in calorimeter will give off varying amounts of heat – and you’re willing to convert their carbon, hydrogen, and oxygen molecules into another form with less energy – water and carbon dioxide which, if burned, produce very little heat – it’s a fair trade! The ETC is simply the vehicle that allows our body to make the switch.
In a car, by contrast, it’s much simpler. The engine combusts the hydrocarbon (e.g., gasoline) directly and in one flash liberates the heat contained within the hydrogen-carbon and carbon-carbon bonds in exchange for carbon dioxide, water vapor, and a few other things.
If you take a look at the figure, below, you’ll get a sense of the moving pieces involved in this cyclic transfer process. Molecules shuffle back and forth, around the cycle, and kick off spent carbon (carbon dioxide, termed “waste”) and reducing agents (e.g., conversion from NAD+ to NADH) for the ETC.
Where do the ketones come in?
In the absence of acetyl CoA (several ways this can happen, including substrate shortage, as I’m describing here) we evolved a cool trick. Our liver can make – out of fat or protein, though we much prefer to use fat so we can spare our protein and prevent severe muscle wasting – something called beta-hydroxybutyrate, one of the 3 ketone bodies I described above.
B-OHB and acetoacetate (see figure below from this paper by Cahill and Veech, 2003) are produced by the liver from long and medium chain fatty acids and released into the bloodstream.
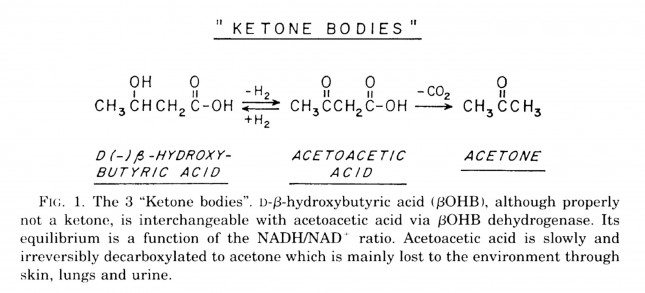
Acetoacetic acid and B-OHB live in reversible equilibrium (on the left), but once acetoacetate is converted to acetone (on the right) there’s no going back.
Now take a look at the figure below, from this 2001 paper. This is another rendition of the figure above showing the Krebs Cycle, but here you can see where B-OHB and acetoacetate enter the picture.
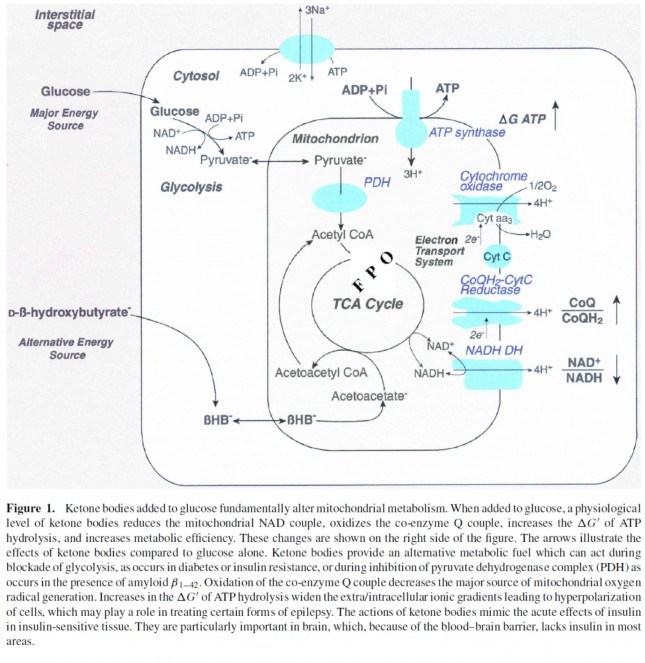
The reason a starving person can live for 40-60 days is precisely because we can turn fat into ketones and convert ketones into substrate for the Krebs Cycle in the mitochondria of our neurons. In fact, the more fat you have on your body, the longer you can survive. As an example of this, you may want to read this remarkable case report of a 382 day medically supervised fast (with only water and electrolytes)! If we had to rely on glucose, we’d die in a few days. If we could only rely on protein, we’d live a few more days but become completely debilitated with muscle wasting.
The graph below, also from the Cahill and Veech paper, shows the blood chemistry of a person starving for 40 days. Within about 3 days, a starving person’s level of glucose stops falling. Within about 10 days they reach a steady-state equilibrium with B-OHB levels exceeding glucose levels and offsetting most of the brain’s need for glucose. In fact, the late George Cahill did an experiment many years ago (probably would never get IRB approval to do such an experiment today) to demonstrate how ketones can offset glucose in the brain. Subjects with very high levels of B-OHB (about 5-7 mM) were injected with insulin until glucose levels reached 1 mM (about 19 mg/dL)! A normal person would fall into a coma at glucose levels below about 40 mg/dL and die by the time blood glucose reached 1 mM. These subjects were completely asymptomatic and 100% neurologically functional.
The last point I’ll make on the starving patient is that, as you can see in the figure below, the glucose level normalizes at about 65-70 mg/dL (about 3.7 mM) within days of fasting, despite no sources of exogenous glucose. Why? Because with so much fat being converted into B-OHB and acetoacetic acid by the liver, a significant amount of glycerol (the 3-carbon backbone of triglycerides) is liberated and converted by the liver into glycogen. As an aside, this is why someone in nutritional ketosis – even if eating zero carbohydrates – still has about 50-70% of a normal glycogen level, as demonstrated by muscle biopsies in such subjects.
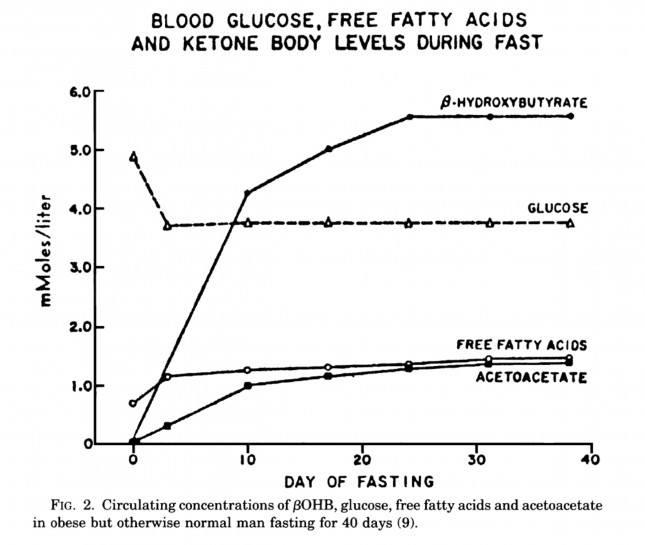
Take home message #3: We evolved to produce ketone bodies so we could not only tolerate but also thrive in the absence of glucose for prolonged periods of time. No ability to produce ketone bodies = no human species.
Last point of background: Everything I’ve just presented is based on data from starving subjects. If one restricts carbohydrate intake, typically to less than about 20-50 gm/day (dependent on timing and carbohydrate composition), and maintains modest but not high protein intake (because protein is gluconeogenic – i.e., protein in excess will be converted to glycogen by the liver), one can induce a state referred to as “nutritional ketosis” with similar physiology to what I’ve just presented without resorting to starvation. Why you’d do this is something I will discuss later.
One other housekeeping issue: Ketosis versus DKA?
In a separate post, I explained the difference between nutritional ketosis (NK) and diabetic ketoacidosis (DKA). If this distinction is not clear, I’d suggest giving this separate post a quick skim for a refresher. DKA is a pathologic (i.e., harmful) state that results from the complete or near absence of insulin. This occurs in the setting of type 1 diabetes or very end-stage type 2 diabetes, and often as the result of a physiologic insult (e.g., an infection) where the patient is not receiving sufficient insulin to bring glucose into his cells. A person with a normal pancreas, regardless of how long he fasts (including the fellow I reference above who fasted for 382 days!) or how much he restricts carbohydrates, can not enter DKA because even a trace amount of insulin will keep B-OHB levels below about 7 or 8 mM, well below the threshold to develop the pathologic acid-base abnormalities associated with DKA. Let me reiterate, it is physiologically impossible to induce DKA in anyone that does not have T1D or very, very, very late-stage T2D with pancreatic “burnout.”
Embarrassing admission: I remember exactly where I was sitting in a clinic at Johns Hopkins in 2002 explaining to (admonishing, really) a patient who was on the Atkins diet how harmful it was because of DKA. I am so embarrassed by my complete stupidity and utter failure to pick up a single scientific article to fact check this dogma I was spewing to this poor patient. If you’re reading this, sir, please forgive me. You deserved a smarter doctor.
In Part II of this post I’ll tackle the questions I know folks still have on their mind (below). Until then, re-read this post to make sure you really understand this physiology. You’re already 10 steps ahead of the next person.
- Is there a “metabolic advantage” to being in ketosis?
- Are there dangers of being in ketosis?
- What are the most important things you need to know about getting into (or staying in) ketosis?

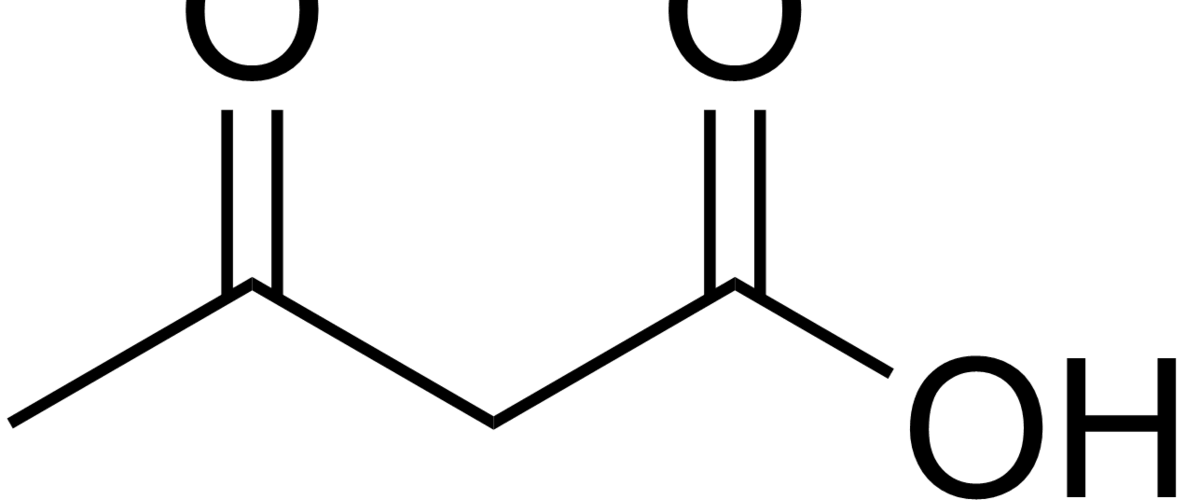

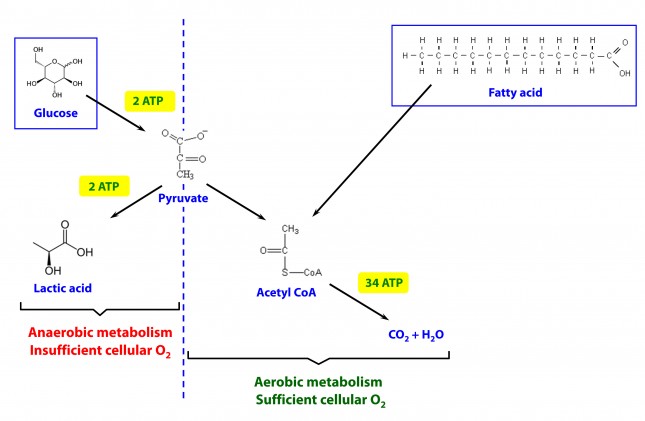




Please clarify how you get 2 ATP from reducing pyruvate to lactate. Thanks.
See this figure for glycolysis: https://en.wikipedia.org/wiki/File:Glycolysis2.svg
Note that part of this process requires ATP, while part of it liberates ATP. The net, however, is a gain of ATP.
Peter, The cited Wickipedia pathway shows glucose to pyruvate, and that gives net 2 ATP. But your figure shows 2 ATP in the step from pyruvate to lactate.
Both happen. Glucose to pyruvate yields 2 (net) ATP. If pyruvate does not enter the mitochondria to become acetyl CoA (not enough cellular oxygen), then it converts to lactate for an additional 2 ATP. Hence, aerobic metabolism is much more efficient, but anaerobic still gives you something.
My question is *how.* Pyruvate is *reduced* to lactate, regenerationg NAD+, which can then feed back into glycolysis to produce more ATP. Is that what you mean? But you still need more glucose to get more ATP.
It’s not really a gain in energy, because you’re sacrificing an NADH (worth about 2 ATP) in order to regenerate the NAD+. But the diagram makes it look as if you get ATP directly.
I don’t want to belabor this, but I’m really puzzled.
In that sense, there is no such thing as a gain in energy, as the laws of thermodynamics tell us. The ATP (the energy we want) is generated by reducing pyruvate (NADH + H+ –> NAD+) to lactate, which liberates CO2 and ATP
It doesn’t seem as though most of the “Biggest Loser” participants found significant long term ketosis. Some have kept the weight off but most are starting to gain it back. I didn’t look closely but it doesn’t appear that any used low carb as a way to lose weight. Sorry if this comment is not in the appropriate section but I wasn’t sure where to put it and I thought it was interesting.
https://today.msnbc.msn.com/id/40423712/ns/today-entertainment/t/biggest-loser-where-are-they-now/#.ULTAfo77A8F
BL patients were on very low calorie diets, but not specifically ketogenic, so I suspect they were not in ketosis. As to why they regain weight, that’s an entirely separate (and longer) discussion, but speaks to the reality of weight loss: calorie restriction have very low long-term success.
As usual, another great post Peter. So my question is from an athletes point of view. If I am in Ketosis and I am a distance runner, will consuming carbs like a gel or SS (during a race) help boost the Krebs Cycle during an 18, 30 or 50 mile run to further burn fat for energy? The one thing I am not sure of is if the carb is used up by the muscle or it is actually transferred to the mitiocondria to keep the Krebs Cycle buring fat to produce said energy
If in ketosis, you’re better off consuming a carb during the event that will have the lowest impact on insulin levels to have he least interference with ketone production. This is one reason I prefer SS for in-race glycogen top-off over, say, a conventional glucose source.
Understood. I am not a fan of the gut churn/cramp up during a race when a glucose source is trying to digest. Not sure if this is the fourm to discuss this but, what about taking a free form amino acid/s to boost energy levels along with SS? I know amino acids can be converted via the Krebs’ cycle to glucose for energy or for storage as glycogen and fat. So during times of increased stress due to trauma, exercise, starvation and/or disease states, amino acids can be catabolized into intermediates to produce energy.
At most a few gm of BCAA might help, but not sure much more than that is needed for the type of exercise you’re doing.
This article has me scratching my head a bit?
https://www.dailymail.co.uk/health/article-2238842/Could-elixir-hold-key-weight-loss-Experts-hope-itll-treat-diabetes-epilepsy-Alzheimers.html
Ahhh…you’re getting a bit ahead. Yes, I was planning to address this in a subsequent post (maybe part 3)? I know Kieran Clarke and Richard Veech personally, and am very familiar with Delta G (ketone ester). I will get to this eventually.
Sounds like a real boon to us low carb types. I would love to get some.
Hi Peter –
Q: If you are in true nutritional ketosis, might it be harmful to the brain to drink alcohol as this will halt ketone production and at the same time, not provide glucose. IOW, if one is relying on ketone bodies for fuel, could halting their production without a concomitant intake of carbohydrate be harmful?
I guess it depends how much alcohol. Even in full-fledged ketosis, your brain still has access to glucose, so you’re never exclusively dependent on B-OHB.
My apologies if this is not directly related to your article, which was very straight forward and easy to follow, thank you (Very interesting about the liver still being 50%-70% full in ketosis. The body must trigger the generation of ketones before al the glucose is drained from your body 🙂 I wonder what the threshold is for that…). But I wonder if you had any comment on the following ketogenic issues (if not I understand your time is quite limited).
– Various sources claim the ‘butter’ range for nutritional ketosis is around 3mmol. I seem to hang around 0.5-1.0. I finally got a meter and have been experimenting with the timing. Ketosis has done wonders for my endurance but I was surprised after a 24-fast with a 5 1/2 hour mountain run in between I was surprised to see my ketone levels only at 0.2 right before dinner ! The highest I have had is 1.0 after a 24 hr. fast with only a 2 hr run in the morning. Is there some credibility to the idea that you become for ‘efficient’ using ketones over time so you body produces less ? Or does that mean I still don’t have things tweaked ? (Insulin resistance? Never had any metabolic syndrome markers before embarking on the diet).
– Has their been any research in regards to ketosis vs. mental disorders like anxiety attack ? I have a friend who is on Paxil and tried to go ‘ketogenic’. They got “pretty” good at cutting out carbs but it seems whenever things finally seemed to start happening it triggers the beginnings of a panic attack so they would dive for the carbs and it would clear up (low glucose ?). I wonder if it is harder to get over the ‘hump’ when someone has low serotonin levels.
– I keep reading about thyroid issues vs. ketosis. I have another friend who is on synthetic hormone and when they tried to go ketogenic their T3 levels took a dive (TSH went up) which then resulted in their LDL to sky rocket (as I have read low thyroid means you cannot clear out the cholesterol as well ? Not sure about this). Do you know of any additional research done with ketosis/starvation and thyroid reactions ? I think it makes sense T3 would drop since there is less oxidative stress on the body.
Thanks so much for your time,
Jason
I’ll address at least a subset of these in part II.
Jason, on the thyroid issue, this panel discussion on safe starches from the Ancestral Health Symposium gets into the low carb and thyroid issue pretty well. Dr. Ron Rosedale challenges the position taken by others that a change in thyroid values with a low carb diet is indicative of pathology. His position is that it’s adaptive.
https://vimeo.com/52872503
There’s also a paper in the journal, Medical Hypothesis (2004) 62, 871-75, which I find most cogent that describes the metabolism of dietary carbohydrate as highly thyroxin demanding, and that T3 especially is carbohydrate dependent. So therefore, reductions in dietary carbohydrate reduce the requirement for T3. This paper discusses studies in which reductions in T3 were not followed by increases in symptoms or signs of thyroid deficiency.
I have been treated for hypothyroidism for quite a long time, and am currently on no medical support for thyroid imbalance since eating VLC. My numbers became almost hyper-thyroid looking, although I had no symptoms. My conclusion is that thyroid tests become as difficult to interpret as some other tests (like LDL particle) under the conditions of ketosis.
“My conclusion is that thyroid tests become as difficult to interpret as some other tests (like LDL particle) under the conditions of ketosis.”
Thyroid tests are difficult to interpret regardless of ketosis. My own endocrinologist has called the failure to recognize this the single biggest mistake in the history of medicine:
https://www.amazon.com/gp/product/1578263875/ref=ox_sc_act_title_4?ie=UTF8&smid=ATVPDKIKX0DER
Thanks a lot Lorraine and Bill, those were useful links.
Unfortunately my friend with hypothyroidism did not do a thyroid test right before going on the diet so the doctor is blaming the elevated TSH levels on the diet. I am not sure if the high TSH levels were already present beforehand. It certainly makes sense that T3 levels would drop with less oxidative stress and the body would adapt to that.
I get the feeling some doctors just have the formula of high TSH = add more synthetic hormone. I am not qualified to argue with that but it sounds awfully close to the high cholesterol = need statins argument.
Anyway, thanks again.
Jason
Hi Jason,
As I’ve also written further down. I had anxiety attacks too and felt somewhat depressed in the adaption phase. After I started supplemented with magnesium it completely went away and my mood improved. This could simply be a general adaption to ketosis too, I can’t say for sure if one or the other was the biggest factor. I’m supplementing with less magnesium now and continue to feel great.
Could you please comment on fiber intake? Do you find fiber supplements necessary or desirable? If so, which ones?
I get more than enough from my vegetables.
HI Peter,
What a great post – I feel like it’s really ‘closing the circle’ for me on so much that I’ve read on your website.
I wanted to (humbly) suggest a sub-topic that could be addressed in your part 2. Perhaps something on the impact of an intermittent high-carb meal on one’s ketotic state. For me, this would help in thinking about the practicality of a low-carbohydrate diet. I’m often able to keep very stringently to low/no carbs, but there are some situations where this just isn’t possible (usually for social reasons). I’d really love to better understand the process by which an intermittent high-carb meal can ‘kick’ you out of nutritional ketosis, the threshold (i.e. how much carb will take you out of ketosis), and how long it takes to get back in.
Thanks!
I’ll try to remember to include this in the final part. Short answer is it highly depends on the quality of the carbs and the timing of ingestion (e.g., right after a long workout or just before bed). Candy bar vs. almonds. These factors, and others, make all the difference in the world.
Mark Sisson covered some good info related to this in one of Jimmy Moore’s “Ask the Low Carb Experts” podcasts. The title was “Ketosis: Devil or Angel.” (You can get it for free from iTunes or probably straight from Jimmy’s site.)
Mark explained that once you’ve been fat/keto-adapted for a while, your body has the “metabolic machinery” in place to move more easily in and out of ketosis than, say, someone jumping into low-crab from the standard American diet. Once you build that cellular machinery (especially additional mitochondria) and your body is sort of “trained” to run on fat, an occasional high-carb meal isn’t that big a deal. (Depending, of course, on *why* you’re ketotic — like if you’re trying to treat/control a serious condition.) There seems to be some dispute about how long it takes to get back into ketosis after a high-carb meal or an all-out binge. Sisson seems to think it doesn’t take that long because you still have the basic machinery in place and your body overall is still fat-adapted. Others (don’t recall exactly who, but it might have been Stephen Phinney or another LC researcher) think it takes a lot longer.
I do a fair amount of business travel, with customer-facing lunches and dinners. A pleasant smile works wonders – “No, thanks – I’m fasting today. Please – go ahead.” And I sip my bottled water. And smile all the way – because I feel great.
@Canuck
i , like amy, have read both dr. volek/phinney and mark sisson.
i had an all out three days binge while in ketosis and it took me more than two weeks to get back on track fully. i had all my muscles full of water, well swollen and my energy level was extremely low. my body switched straight away from fat burning to glycogen, therefore when i re-started eating low carb i could not even do a quarter of the sport i did until three days before as my body expected gucose but i was just giving fat and gluconeogenesis is not so immediate.
another time i had one day all carbs and it took me about one week.
you can try for yourself, but it is not pleasant at all.
i have also overeaten for two days “allowed food”, in the form of cream, fatty cheese and nuts, but i found no water retention, little energy reduction and back on my way in two days. only hunger ( leptin-grehlin i suppose) was a little awry.
alan
Thanks for the post Dr. Attia. You may be already planning to cover this topic, but one thing I’ve been wondering about is whether there is any disadvantage (performance, metabolic, or otherwise) to being on the border of ketosis and sugar-burning vs. solidly in one camp or the other. I’ve had success with weight loss by reducing carbs to 50-100, but I’m only just over half-way through my planned loss, and I’m trying to plan ahead for any walls I might encounter on my way.
Probably depends very highly on the individual.
Hey Dr. Attia,
“Long time listener, first time caller,” as they say. I discovered your blog through Robb Wolf, after he mentioned your (insanely incredible) series on cholesterol. I’ve been devouring your site as fast as I can…kind of like a pat of butter on a spoon all by itself, which I’ve been known to devour, too!
Anyway, I just wanted to say that you have a real gift for attacking the nitty-gritty science head-on but also translating what it all *means* it comes to what we should and shouldn’t put in our mouths. If I was one tenth as prolific as you are, I’d be dangerous. (In a good way.)
I just finished up a master’s in nutrition, and I thought the biochem was going to be the most challenging part of it. Turns out I was FASCINATED by it all and can’t get enough, so your site is truly a refuge in the sea of misinformation that’s out there about nutrition and health. You’re exactly right about Richard Feynman — you can’t argue with the science. And when it comes to the unfathomable mess we’ve gotten ourselves into with the utterly nonsensical low fat, whole grain paradigm, the only way we’re gonna dig ourselves out is for the science to win. Not the politically correct recommendations, the moral grandstanding, or even what we *wish* were true or sounds logical on paper. (I mean, let’s face it, to someone who knows nothing about biochem and physiology, it *makes sense* that fat should make you fat…) So I’m especially grateful to you and Gary Taubes for starting NuScI. Can’t wait to see where things go.
And one last thing…sorry to talk your ear off, but I want to make sure I tell you that the quote below might just be the single greatest thing I’ve read on a nutrition/health blog — and I read *a lot* of them! Kudos for your humility and intelligence. If only all doctors had the curiosity to question the “facts.” You’re a rare breed!
“Embarrassing admission: I remember exactly where I was sitting in a clinic at Johns Hopkins in 2002 explaining to (admonishing, really) a patient who was on the Atkins diet how harmful it was because of DKA. I am so embarrassed by my complete stupidity and utter failure to pick up a single scientific article to fact check this dogma I was spewing to this poor patient. If you’re reading this, sir, please forgive me. You deserved a smarter doctor.”
Amy, I’m so delighted the world of nutrition professionals is now one person richer by your addition. Thanks so much for kind words and I’m delighted to play even a tiny part in your ongoing education.
Peter, it is such a privilege to have access to your work. I just wonder how many hours of sleep you get.
I am really pleased you are doing something on ketosis.
I reduced my carbs signicantly a year ago and have lost 50% of my body fat and reduced my weight by 13kg. Since starting a full ketogenic diet 3 months ago, the best thing has been a 65% reduction in my triglycerides and a 35% increase in my HDL. I know this isn’t the whole story but here in New Zealand we don’t have NMR.
As a typical obsessive physician, I have been regularly checking my ketones and blood sugars and am really impressed how tightly controlled blood sugars are despite a very low carb intake and even after prolonged exercise. Physiology and biochem have never been so interesting! I had a steroid injection in an injured shoulder recently. I was really surprised by my blood sugar which shot up to around 8.2mmol/l and stayed high for about 2 weeks. I would have expected this to increase insulin levels and kick me out of ketosis but my ketones stayed up throughout this time although lower than average. Turns out steroids exert their effects on blood sugar by inducing insulin resistance in the liver and muscles, but also prevent the ketone-supressant effect of insulin.
A thing that has really surprised me about being in ketosis is the mental/mood effects – specifically euphoria which kicks in at elevated ketone levels -seems to be above about 2 mmol/l. It is quite noticeable and almost disruptive to normal work. Conversely, I notice definitely lower mood when my ketones are low, typically early in the day. This effect does not seem to be diminishing with time.
Anyway, keep up the good work – it is really appreciated.
Andrew, thanks so much sharing your experience. Great to know the pool of physicians out there who understand this is growing. As for sleep…probably not enough but MUCH more than residency!
Peter:
You mention reading 5 or 6 scientific articles a week. How do you find these articles and how do you separate the good from the bad?
Mark
Actually, my pile-up list is about 20 papers per week, but I try to winnow it down to 5 to 6 based on topics I need to learn more about and the quality of the work. I try to assess this quickly be reading the abstract and methods, plus knowing something about the author.
Hi Peter and thank you for a wonderful post, so well explained for someone like me with no biochemical or medical background. I have T2D with normal (non-diabetic) blood glucose and an HbA1c of 5.0 and have been keto-adapted for about 6 months or more, testing B-OHB since early June. Results were consistently over 2.0 at any time of the day with a highest recording of 3.4. So as the strips are expensive and I was keeping an accurate diary, recording a KR of 2.2-2.3 consistently, I stopped testing. This afternoon, after 6 weeks without testing, I bought a packet of 10 beta-ketone strips and tested, getting a horrible fright when the result was 6.3.
So I was very relieved to read your statement about only T1s and very late stage T2s being at risk of DKA. Thank you for the timeliness of that statement. 🙂
This series is very exciting, and although I’ve tried to read a lot about ketosis, I had several light-bulb moments while reading part 1. Bring on part 2!
Ann, you must be eating very few overall calories to reach those levels? The reason I assume this is because with 30-50 gm of carb and, say, 100 gm of protein in your diet, levels this high would very difficult to achieve. I must admit, I’m kind of jealous! As you’ll see in part II, you are achieving benefits from ketosis that most people living at 1 to 2 mM do not.
Amazing but in all honesty … how can you understand all this … what our body does …, and still say evolved with a straight face. Cracks me up.
Excellent write up yet again.
@ Andrew,
I experimented with being in NK for a few weeks some time ago, but got out of it. My number one reason for wanting to do it again is definitely the oh-so-happy mood – euphoria as you call it is the right word. I was so balanced. Unexpected, delightfully good mood 🙂
Peter, I’ve learned more from your blog than I could have ever imagined.
Thank you! Keep up the amazing work.
P.s Spelling error in paragraph 8 Glucose-6-phophatase = Glucose-6-phosphatase
Great catch, Greg. Thank you.
Peter-
This is an excellent article. Thanks for the great work. I am loving your blog. -Greg
Thanks very much, Greg. Very glad Stuart was able to re-connect us.
Thanks, Doc. A great explanation of how we get to ketones. I think that if people reading your post are a little blown away by the metabolic pathways, maybe they can read Dr. Eades great conceptual article on metabolic ketosis from his blog first (or read it afterward, and then come back again for a second read on this one). I’ve been hoping for a couple of years now that someone would do a really comprehensive explanation of the biochemistry of ketosis for the lay person. As much as I’m a huge fan of Drs. Volek and Phinney, I’ve been disappointed that they’ve not had at it with more detail, especially in Art and Science of Low Carb Living, which is also aimed at clinicians. Now, with the combination of the Eades post, the degree to which Volek and Phinney do get into it, and here, people can fully wrap their brains around how it works and why it’s normal physiology.
One of the things I actually still hear about as a disadvantage or danger of ketosis, is that ketones are “partially burned fats” or “inefficiently burned fats”, whatever that means. I’ve tried to do a lot of ‘splainin of this concept, which seems to have gotten started back in the day of early body building circles and has persisted. In any event, you can’t really explain what’s wrong with that statement unless you look at the metabolic pathways and show folks that ketones are made *after* beta oxidation, which means that the fatty acids have been completely metabolized. Getting folks accustomed to seeing metabolic pathways by posting them here is enormously helpful in the end, even if initially folks go to total brain block when they first see one.
Warning: Nit Pick Coming Up – in your second table showing the two routes of glycolysis, which you describe as aerobic and anaerobic, and define as sufficient oxygen vs. insufficient oxygen respectively – sorry, but this makes us exercise physiology types nuts. Just because anaerobic or rapid glycolysis * can* make ATP without oxygen, doesn’t mean there’s insufficient oxygen. Whether or not a substrate is being metabolized oxidatively or glycolytically is determined by all kinds of things irrespective of O2, including rate of demand or product feedback inhibition someplace else in the stream of any particular pathway. To quote Brook and Fahey, in the canon Exercise Physiology, “The early experimentation and terminology has led to some confusion in contemporary physiology……..The terms aerobic (O2) and anerobic (without O2) refer to the test tube conditions used by early researchers to speed up or slow down glycoolysis. In real life, pyruvate and lactate pools are in equilibrium, and the rapidity of glycolysis largely determines the product formed (p 73-4)”. So, I understand that the aerobic/anaerobic nomenclature is more familiar, but it’s completely misleading because it implies that the non-oxidative pathway performs when there’s insufficient oxygen, rather than that we have a pathway that uses oxygen to make ATP, but we also have a pathway that can make ATP without using oxygen. There is also evidence that there’s never insufficient O2, showing that even at VO2max when glycolytic pathways are running full bore, that there’s still at least 2Torr oxygen pressure in the mitochondria. So better terms to use are oxidative or slow glycolysis, and non-oxidative or rapid glycolysis.
Peter, please indulge me more on this aerobic vs. anaerobic thing, because thinking of it more, it’s actually quite important, and the use of those terms is misleading. So the take home is that whether or not glucose runs down to pyruvate or to lactate has nothing to do with availability of oxygen, it has to do with how fast you need ATP. It’s a fast vs. slow issue.
As stated above, even at VO2 max, 2Torr O2 pressure exists in the mitochondria, quite sufficient to continue to produce ATP via the electron transport chain, which does continue, even when ATP is simultaneously being made by rapid (anaerobic) glycolysis…..glucose > lactate. So there’s always enough O2 even at maximal demand for ATP.
Conversely, if the route of glycolysis was oxygen dependent, then there would be no reason for the healthy human to make ATP via rapid (anaerobic) glycolysis at rest. And yet, we do. Certainly you make less lactate at rest than someone with metabolic disease who’s a sugar burner by virtue of not being able to access their fat, but you make it none the less. If the route of glycolysis was related to whether or not sufficient oxygen was available, then you’d never do glucose > lactate at rest when plenty of oxygen is available.
The real reason to change the nomenclature from aerobic to slow glycolysis, and anaerobic to rapid glycolysis is because no matter what the route, there is zero oxidation until you get to the electron transport chain. So glucose to pyruvate is non-oxidative or anaerobic also. So is FFA to Acetyl-CoA, and whatever it is that I can’t now remember amino acids go thru to get to Acetyl-CoA. Even the TCA cycle is anaerobic. Look at your own TCA pathway above. No oxidation until the ETC (one oxygen radical indicated at the ETC cytochrome). So everything north of the ETC -, where AMP and ADP undergo oxidative phosphorylation to ATP – is anaerobic, no matter what your route or substrate. Therefore to say that glucose > lactate is anaerobic, and glucose > pyruvate is aerobic, is false (because that part of the pathway is also anaerobic, not becoming “aerobic” or oxidative until it gets past the TCA cycle and into the electron transport chain).
The meaningful difference in the routes has to do with the speed with which one needs to get ATP out. So it’s obvious that the aerobic or oxidative, or SLOW, pathways, are just that – slow. There are so many more intermediary substrates that can get hung up by product feedback inhibition in all of the various routes and cycles down to the ETC, and so many more enzymes that can be affected by increases in temperature or decreases in pH. So even though one gets so many more ATP’s per unit of substrate out of the slow pathways, you know, they’re slow, and more prone to log jam. Well, that’s a bummer if you need to sprint to the finish or, if a diabetic for example, to walk across the street. So we have this nifty rapid route that makes ATP without the electron transport chain, and therefore, doesn’t need oxygen (even though there may be plenty of oxygen available). You only get 2 ATP’s out per lactate, but it could make the difference between getting done what the muscle is demanding because that route gets it done fast.
I hope you can understand why I’ve made a pain of myself on this.
Yes, agree with your point and the criticism of my overly simple explanation. I guess one way to think about, though I agree with your assertion about speed, is contrasting 2 people with different VO2 profiles (to power, or some other output metric). The person with the higher VO2 has a greater ability to uptake and utilize O2 at the cellular level, presumably. They would therefore presumably be able to undergo oxidative metabolism under greater energy demand.
Yes, that’s true, but it’s not the point I’m making (or failing to make, lol). Your label on your second figure indicating that “anaerobic” (or non-oxidative, or rapid) glycolysis occurs under the condition of insufficient oxygen is incorrect – that’s the point I’m trying to make. Rapid glycolysis occurs under all conditions of cellular oxygen. You do glucose > lactate all day long, even at rest while you’re reading this, so you’re making lactate right now, even under the condition of sufficient cellular oxygen. Rapid glycolysis is a pathway that just doesn’t happen to use oxygen, even though oxygen may be readily available for use (and is, in fact, simultaneously being used in parallel metabolic pathways like slow glycolysis, or beta oxidation). It’s just a short and quick pathway to crank out some ATP, and it just doesn’t happen to need oxygen to do it. But it is not reflective of insufficient cellular oxygen. So the label is wrong. All the ATP pathways are running all the time, under all conditions (high/low cellular oxygen, rest, exercise etc) – some of them use oxygen to make ATP, and one of them doesn’t need to.
There are, in fact, conditions under which O2 delivery to or utilization by the cell is compromised, and rapid glycolysis becomes more greatly utililzed in those conditions. A couple of these, of course, are high intensity exercise and metabolic disease, but these are not the only conditions under which we do rapid glycolysis. That pathway is used all day long to some degree by healthy people, at rest or in submaximal exercise, under conditions of more than enough cellular oxygen, the rapid glycolysis pathway is just not using that oxygen to make its ATP.
Thanks for your patience.
Peter,
Thanks for your posts.
I read the whole series of cholesterol in 2 nights, my wife was mad at me…
I am following a paleo diet for almost 3 years, and I started IF (I skip 2 meals twice a week) 2 months ago, and as I eat low carb, I may be in the treshold of ketosis.
I write from Barcelona, where this diet is seen as something bizarre (my friends make a lot of funny comments…)
I feel great, but there is one issue; they tell me I am having bad breath. Do you think it can be because of the cetone in the breath?
Might be the acetone. May be transient.
Hi Peter
Quote: Ann, you must be eating very few overall calories to reach those levels? The reason I assume this is because with 30-50 gm of carb and, say, 100 gm of protein in your diet, levels this high would very difficult to achieve. I must admit, I’m kind of jealous! As you’ll see in part II, you are achieving benefits from ketosis that most people living at 1 to 2 mM do not.
Thanks for that reply, Peter. I eat between 1500 and 1700 calories a day, with an F:P:C ratio of around 83:12:5. Protein is around 50 grams a day, carbs 17-25 and saturated and monounsaturated fats make up the rest. I walk 6.25km most mornings, before breakfast and starting out in a fasting state, 12-13 hours since I last ate. I’m relying on Calorie King to calculate the F:P:C ratio for me and I also rely on their database for most of the nutrition data. Oh and I’m actively losing weight. I hope to learn how to make the transition to maintenance without compromising my blood glucose numbers at some stage during your series. 🙂
I’m really looking forward to Part II, and thanks again for a great blog!