When I wrote part I of this post, I naively assumed this would only be a two-part series. However, so many great questions and comments emerged from the discussion that I realize it’s worth spending much more time on this important and misunderstood topic. In terms of setting expectations, I suspect this series will require at least four parts.
So, back to the topic at hand…. (You may want to read or maybe reread part I for a biochemistry refresher before diving into part II.)
Is there a “metabolic advantage” to being in ketosis?
Few topics in the nutrition blogosphere generate so much vitriolic rhetoric as this one, and for reasons I can’t understand. I do suspect part of the issue is that folks don’t understand the actual question. I’ve used the term “metabolic advantage” because that’s so often what folks write, but I’m not sure it has a uniform meaning, which may be part of the debate. I think what folks mean when they argue about this topic is fat partitioning, but that’s my guess. To clarify the macro question, I’ve broken the question down into more well-defined chunks.
Does ketosis increase energy expenditure?
I am pretty sure when the average person argues for or against ketosis having a “metabolic advantage” what they are really arguing is whether or not, calorie-for-calorie, a person in ketosis has a higher resting energy expenditure. In other words, does a person in ketosis expend more energy than a person not in ketosis because of the caloric composition of what they consume/ingest?
Let me save you a lot of time and concern by offering you the answer: The question has not been addressed sufficiently in a properly controlled trial and, at best, we can look to lesser controlled trials and clinical observations to a make a best guess. Believe me, I’ve read every one of the studies on both sides of the argument, especially on the ‘no’ side, including this one by Barry Sears from which everyone in the ‘no’ camp likes to quote. This particular study sought to compare a non-ketogenic low carb (NLC) diet to a ketogenic low carb (KLC) diet (yes, saying ‘ketogenic’ and ‘low carb’ is a tautology in this context). Table 3 in this paper tells you all you need to know. Despite the study participants having food provided, the KLC group was not actually in ketosis as evidenced by their B-OHB levels. At 2-weeks (of a 6-week study) they were flirting with ketosis (B-OHB levels were 0.722 mM), but by the end of the study they were at 0.333 mM. While the difference between the two groups along this metric was statistically significant, it was clinically insignificant. That said, both groups did experience an increase in REE: about 86 kcal/day in the NLC group and about 139 kcal/day in the KCL group (this is calculated using the data in Table 3 and Table 2). These changes represented a significant increase from baseline but not from each other. In other words, this study only showed that reducing carbohydrate intake increased TEE but did not settle the ‘dose-response’ question.
This study by Sears et al. is a representative study and underscores the biggest problems with addressing this question:
- Dietary prescription (or adherence), and
- Ability to accurately measure differences in REE (or TEE).
Recall from a previous post, where I discuss the recent JAMA paper by David Ludwig and colleagues, I explain in detail that TEE = REE + TEF + AEE.
Measuring TEE is ideally done using doubly-labeled water or using a metabolic chamber, and the metabolic chamber is by far the more accurate way. A metabolic chamber is a room, typically about 30,000 liters in volume, with very sensitive devices to measure VO2 and VCO2 (oxygen consumed and carbon dioxide produced) to allow for what is known as indirect calorimetry. The reason this method is indirect is that it calculates energy expenditure indirectly from oxygen consumption and carbon dioxide production rather than directly via heat production. By comparison, when scientists need to calculate the energy content of food (which they do for such studies), the food is combusted in a bomb calorimeter and heat production is measured. This is referred to as direct calorimetry.
Subjects being evaluated in such studies will typically be housed in a metabolic ward (don’t confuse a metabolic ward with a metabolic chamber; the ward is simply a fancy hospital unit; the chamber is where the measurements are made) under strict supervision and every few days will spend an entire 24 hour period in one such chamber in complete isolation (so no other consumption of oxygen or production of carbon dioxide will interfere with the measurement). This is the ‘gold standard’ for measuring TEE, and shy of doing this it’s very difficult to measure differences within about 300 kcal/day.
Not surprisingly, virtually no studies use metabolic chambers and instead rely on short-term measurement of REE as a proxy. In fact, there are only about 14 metabolic chambers in the United States.
A broader question, which overlays this one, is whether any change in macronutrients impacts TEE.
Despite the limitations we allude to in the summary of this review, there is a growing body of recent literature (for example this study, this study, and this study) that do suggest a thermogenic effect, specifically, of a ketogenic diet, possibly through fibroblast growth factor-21 (FGF21) which increases with B-OHB production by the liver.
These mice studies (of course, what is true in mice isn’t necessarily true in humans, but it’s much easier to measure in mice) show that FGF21 expression in the liver is under the control of the transcription factor peroxisome proliferator-activated receptor a (PPARa), which is activated during starvation. Increased FGF21 promotes lipolysis in adipose tissue and the release of fatty acids into the circulation. Fatty acids are then taken up by the liver and converted into ketone bodies. FGF21 expression in liver and adipose tissue is increased not only by fasting but also by a high fat diet as well as in genetic obesity which, according to these studies, may indicate that increased FGF21 expression may be protective. Hence, ketosis may increase TEE either by increasing REE (thermogenic) or AEE (the ketogenic mice move more). Of course, this does not say why. Is the ketogenic diet, by maximally reducing insulin levels, maximally increasing lipolysis (which dissipates energy via thermogenic and/or activity ‘sinks’) or is the ketogenic diet via some other mechanism increasing thermogenesis and activity, and the increased lipolysis is simply the result? We don’t actually know yet.
Bottom line: There is sufficient clinical evidence to suggest that carbohydrate restriction may increase TEE in subjects, though there is great variability across studies (likely due the morass of poorly designed and executed studies which dilute the pool of studies coupled with the technical difficulties in measuring such changes) andwithin subjects (look at the energy expenditure charts in this post). The bigger question is if ketosis does so to a greater extent than would be expected/predicted based on just the further reduction in carbohydrate content. In other words, is there something “special” about ketosis that increases TEE beyond the dose effect of carbohydrate removal? That study has not been done properly, yet. However, I have it on very good authority that such a study is in the works, and we should have an answer in a few years (yes, it takes that long to do these studies properly).
Does ketosis offer a physical performance advantage?
Like the previous question this one needs to be defined correctly if we’re going to have any chance at addressing it. Many frameworks exist to define physical performance which center around speed, strength, agility, and endurance. For clarity, let’s consider the following metrics which are easy to define and measure
- Aerobic capacity
- Anaerobic power
- Muscular strength
- Muscular endurance
There are certainly other metrics against which to evaluate physical performance (e.g., flexibility, coordination, speed), but I haven’t seen much debate around these metrics.
To cut to the chase, the answers to these questions are probably as follows:
- Does ketosis enhance aerobic capacity? Likely
- Does ketosis enhance anaerobic power? No
- Does ketosis enhance muscular strength? Unlikely
- Does ketosis enhance muscular endurance? Likely
Why? Like the previous question about energy expenditure, addressing this question requires defining it correctly. The cleanest way to define this question, in my mind, is through the lens of substrate use, oxygen consumption, and mechanical work.
But this is tough to do! In fact, to do so cleanly requires a model where the relationship between these variables is clearly defined. Fortunately, one such model does exist: animal hearts. (Human hearts would work too, but we’re not about to subject humans to these experiments.) Several studies, such as this, this, and this, have described these techniques in all of their glorious complexities. To fully explain the mathematics is beyond the scope of this post, and not really necessary to understand the point. To illustrate this body of literature, I’ll use this article by Yashihiro Kashiwaya et al.
The heart is studied because the work action is (relatively) simple to measure: cardiac output, which is the product of stroke volume (how much blood the heart pumps out per beat) and heart rate (how many times the heart beats per minute). One can also measure oxygen consumption, all intermediate metabolites, and then calculate cardiac efficiency. Efficiency increases as work increases relative to oxygen consumption.
Before we jump into the data, you’ll need to recall two important pieces of physiology to “get” this concept: the acute (vs. chronic) metabolic effect of insulin, and the way ketone bodies enter the Krebs Cycle.
The acute metabolic effects of insulin are as follows:
- Insulin promotes translocation (movement from inside the cell to the cell membrane) of GLUT4 transporters, which facilitate the flux of glucose from the plasma into the inside of the cell.
- Insulin drives the accumulation of glycogen in muscle and liver cells, when there is capacity to do so.
- Least known by most, insulin stimulates the activity of pyruvate dehydrogenase (PDH) inside the mitochondria, thereby increasing the conversion of pyruvate to acetyl CoA (see figure below).
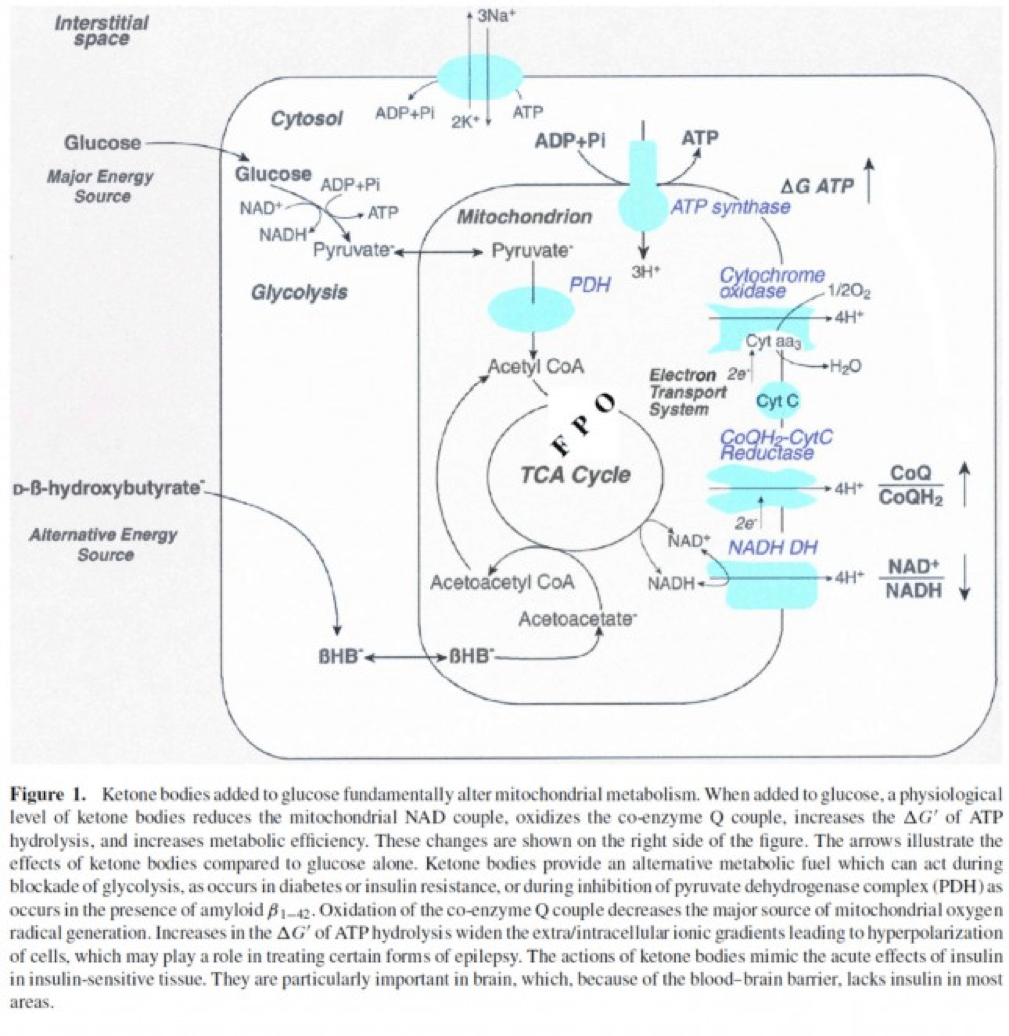
The second important point to recall is that ketone bodies bypass this process (i.e., glucose to pyruvate to acetyl CoA), as B-OHB enters the mitochondria, converts into acetoacetate, and enters the Krebs Cycle directly (between succinyl CoA and succinate, for any biochem wonks out there). I keep alluding to this distinction for a reason that will become clear shortly.
An elegant way to test the relative impact of glucose, insulin, and B-OHB on muscular efficiency is to “treat” a perfused rat heart under the following four conditions:
- Glucose alone (G)
- Glucose + insulin (GI)
- Glucose + B-OHB (GK)
- Glucose + insulin + B-OHB (GIK)
In fact, that’s exactly what this paper did. Look at what they found:
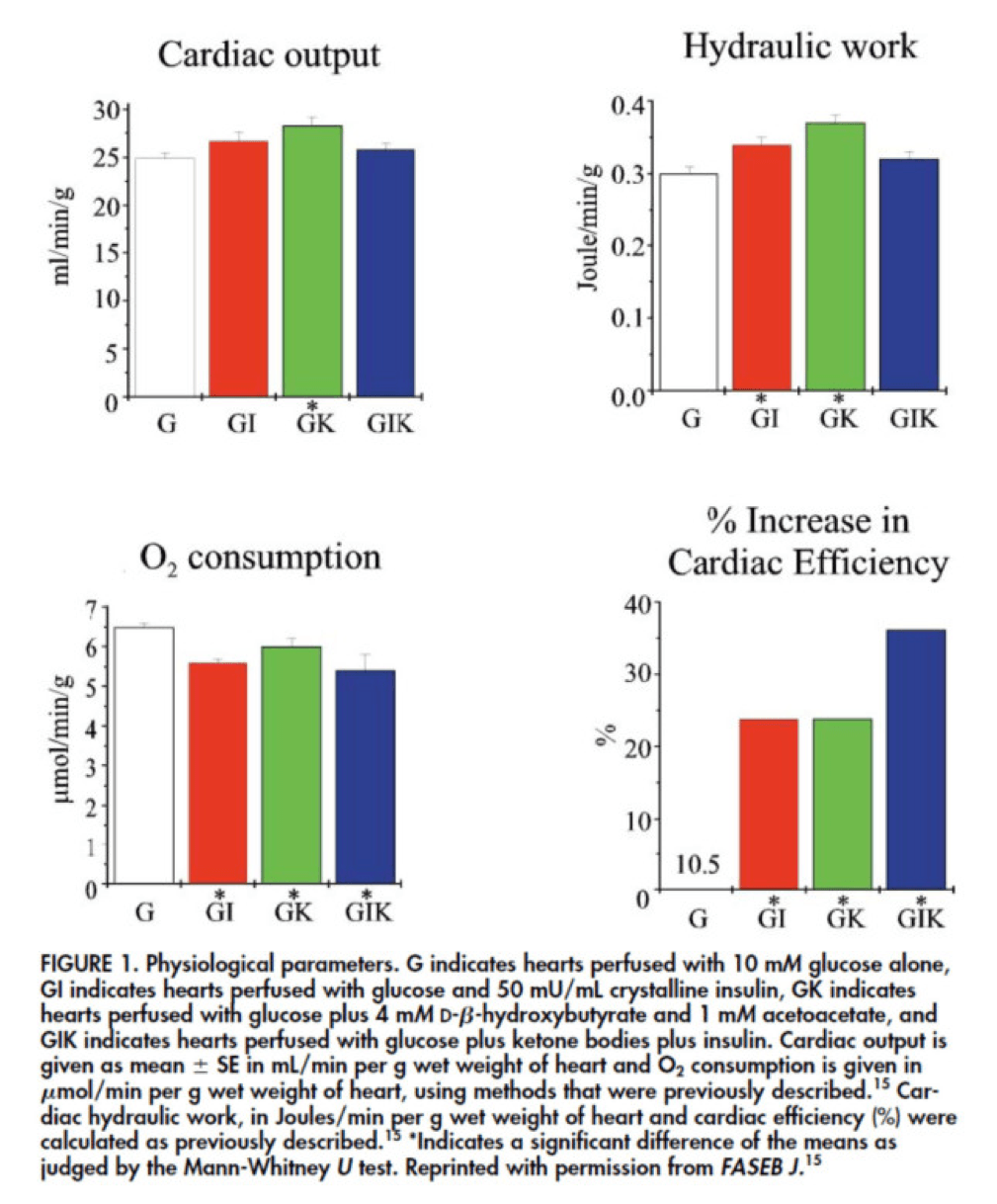
The upper two graphs in this figure show similar information, namely the response of cardiac output and hydraulic work to each treatment. (Cardiac output is pure measurement, as I described above, of volume of blood displaced per unit time. Hydraulic work is a bit more nuanced; it measures the mechanical work being done by the fluid.)
Adding insulin to a fixed glucose (GI) load increases both cardiac output and hydraulic work, but it’s only significant in the case of hydraulic work. Conversely, adding B-OHB to glucose (GK) increases both cardiac output and hydraulic work significantly. Interestingly, combining insulin and B-OHB with glucose (GIK) increases neither.
Oxygen consumption was significantly reduced in all arms relative to glucose alone, so we expect the cardiac efficiency to be much higher in all states. (Why? Because for less oxygen consumption, the hearts were able to deliver greater cardiac output and accomplish greater hydraulic work.)
The figure on the bottom right shows this exactly. If you’re wondering why the gain in efficiency is so great (24-37%), the answer is not evident from this figure. To understand exactly how and why adding high amounts of insulin (50 uU/mL) or B-OHB (4 mM) to glucose (10 mM) could cause such a step-function increase in cardiac efficiency, you need to look specifically at how the concentration of metabolic intermediates (e.g., ATP, ADP, lactate) varied in the rat heart cells.
This is where this post goes from “kind of technical” to “really technical.”
The figure below presents the results from this analysis. The height of the bar shows the fold-increase for each of the three treatments relative to glucose alone. To orient you, let’s look at a few examples. In the upper left of the figure you’ll note that GI and GIK both significantly increase glucose concentration in the cell, while GK does not. Why? The GI and GIK treatments both increase the number of GLUT4 transporters translocated to the cell surface so more glucose can flux in. GK does increase glucose concentration, but not significantly (in the statistical sense).
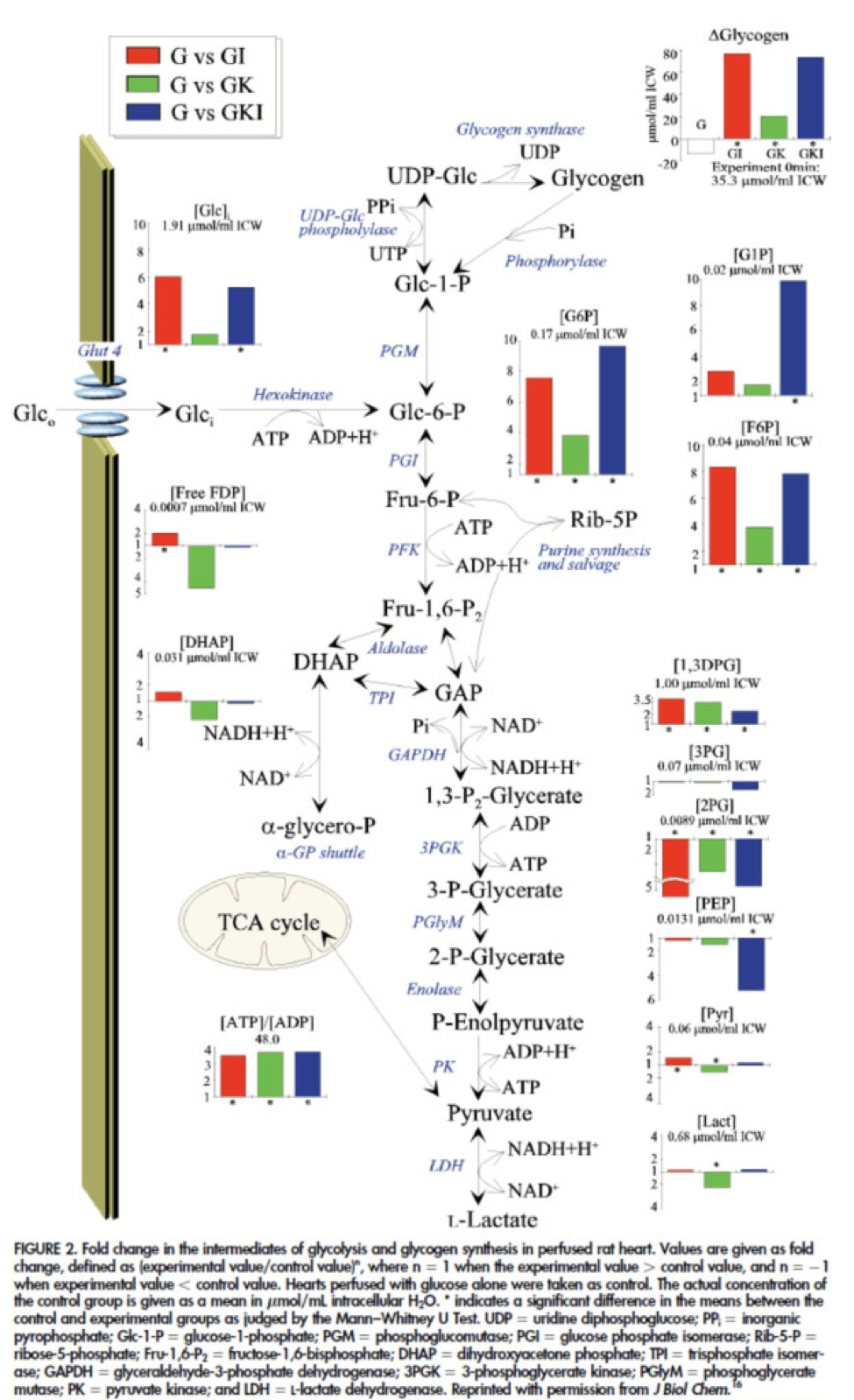
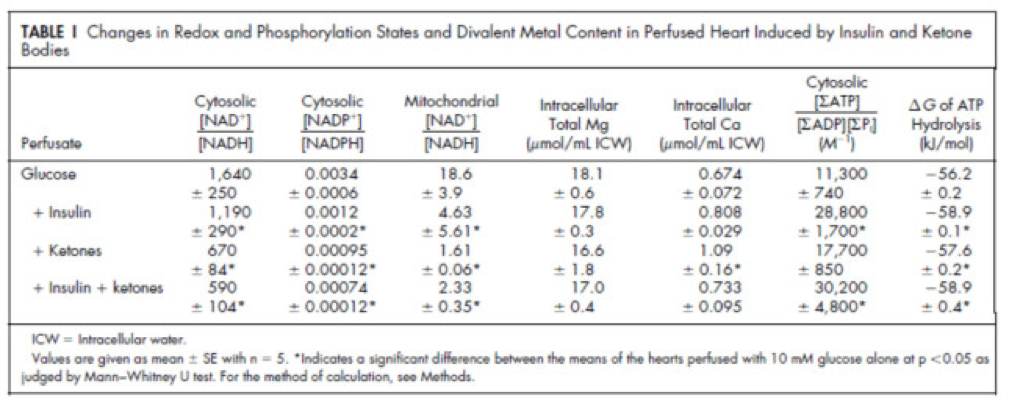
Table 1 from this paper, below, summarizes the important changes from this analysis. In particular, look at the last column, the Delta G of ATP hydrolysis.
I was really hoping to write this post without ever having to explain Delta G, but alas, I’ve decided to do it for two reasons:
- To really “get” this concept, we can’t avoid it, and;
- The readers of this blog are smart enough to handle this concept.
Delta G, or Gibbs free energy, is the “free” (though a better term is probably “available” or “potential”) energy of a system.
Delta G = Delta H – Temperature * Delta S, where H is enthalpy and S is entropy. The more negative Delta G is, the more available (or potential or “free”) energy exists in the system (e.g., a Delta G of -1000 kcal/mol has more available energy than one of -500 kcal/mol). To help with the point I really want to make I refer to you this video which does a good job explaining Gibbs free energy in the context of a biologic system. Take a moment to watch this video, if you’re not already intimately familiar with this concept.
Now that you understand Delta G, you will appreciate the significance of the table above. The Gibbs free energy of the GI, GK, and GIK states are all more negative than that of just glucose. In other words, these interventions offer more potential energy (with less oxygen consumption, don’t forget, which is the really amazing part).
To see what the substrate-by-substrate changes look like across the mitochondria and ETC, look at this figure:
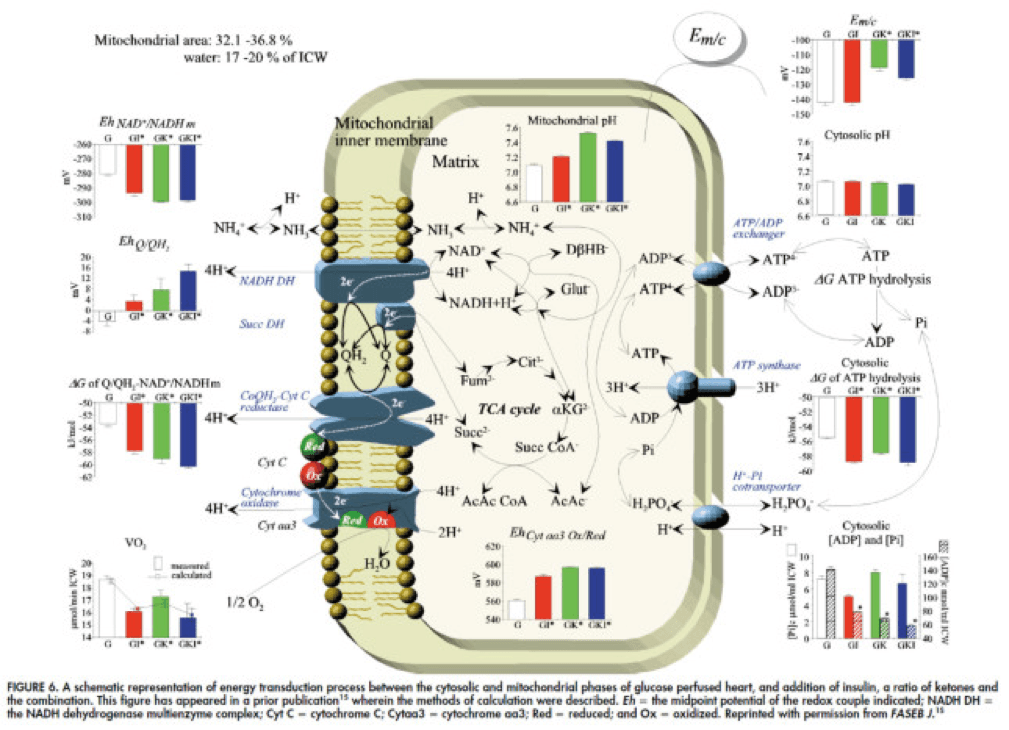
Though it is by no means remotely obvious, what is happening above boils down to two major shifts in substrate utilization:
- In one step the reactants NADH/NAD+ become more reduced (in the chemical sense), and;
- In another step the reactants CoQ/CoQH2 become more oxidized (in the chemical sense).
These changes, taken together, widen the energetic gap between the states and, in turn, translates to a higher (i.e., more negative) Delta G which translates to greater ATP production per unit of carbon.
Additional work, which you’ll be delighted to know I will not detail here, in fact shows that on a per carbon basis, B-OHB generates more ATP per 2-carbon moiety than glucose or pyruvate. As an aside, this phenomenon was first described in 1945 by the late Henry Lardy, who observed that sperm motility increased in the presence of B-OHB (relative to glucose) while oxygen consumption decreased!
Is there a reason to prefer GK over GI?
Yes. Recall that ketones make their way onto the metabolic playing field without going through PDH. Adding more insulin to the equation forces more pyruvate towards PDH into acetyl CoA. While B-OHB “mimics” the effect of additional insulin, it does so in a much cleaner fashion without the complex cascade of events brought on by additional insulin (e.g., decreased lipolysis) and, perhaps most importantly, avoids the logjam of impaired PDH due to insulin resistance (I’ll come back to this point in a future post when I address Alzheimer’s disease and Parkinson’s disease). In essence, B-OHB “hijacks” the Krebs Cycle via a slick trick that lets it bypass the bottleneck, PDH. All the glucose and insulin in the world can’t overcome this bottleneck. It’s truly a privileged state and a remarkable evolutionary trick that we can utilize B-OHB.
Back to the original question…
Clearly, in the highly controlled setting of a perfused rat heart, ketones offer an enormous thermodynamic advantage (28%!). But what about in aggregate human performance? There is no reason to believe that therapeutic levels of B-OHB (either through nutritional ketosis or by ingesting ketone esters) would increase anaerobic power, since the anaerobic system does not leverage the Delta G improvement I’ve outlined here. Same is true for muscular strength. However, there is reason to believe that aerobic capacity and muscular endurance could be improved with sufficient B-OHB present to compliment glucose.
It turns out this has been demonstrated repeatedly in subjects ingesting ketone esters, developed by Dr. Richard Veech (NIH) and Dr. Kieran Clarke (Oxford). Because the results of their work have not yet been published, I can’t comment much or share the data I have, which they shared with me. I can say the ingestion of B-OHB in the D-isoform (the physiologic isoform), resulting in serum levels between 4 and 6 mM, did lead to significant increases in aerobic power and efficiency in several groups of elite athletes (e.g., Olympians) across multiple physical tasks maximally stressing the aerobic system.
Once published, I believe these studies will be a real shot across the bow of how we view athletic performance. It is very important to point out, however, that these studies don’t exactly address the most relevant question, which has to do with nutritional ketosis. In other words, ingesting ketone esters to a level of 4 to 6 mM might not be the same as de novo producing B-OHB to those levels. But, such trials should be forthcoming in the next few years. Personally, I am most eager to see the results of a ketone ester alone versus nutritional ketosis versus combination treatment, all to the same serum level of B-OHB.
The Hall Paradox
For the really astute readers, you may be saying, “Waaaaaaaait a minute, Peter…if ketones increase Gibbs free energy while reducing oxygen consumption, should this imply TEE goes down?” You’re right to ask this question. It was the first question I asked when I fully digested this material. If each molecule of B-OHB gives your muscles more ATP for less oxygen, you should expend less not more energy at the same caloric intake, right?
I was discussing this with Kevin Hall at NIH, an expert in metabolism and endocrinology. Kevin pointed out the error in my logic. I failed (in my question) to account for the energetic cost of making the ketones out of fat. Remember, in the experiments described above, the B-OHB is being provided for “free.” But physiologically (i.e., in nutritional ketosis or even starvation), we have to make the B-OHB out of fat. The net energy cost of doing this is actually great. According to Kevin, it is not generally appreciated how making ketones from fatty acids affects overall energy efficiency. Nevertheless, this can be examined by comparing the enthalpy of combustion of 4.5 moles of B-OHB, which is about -2,192 kcal, with the enthalpy of combustion of 1 mole of stearic acid (about -2,710 kcal) that was used to produce the 4.5 moles of ketones. Thus, there is about 20% energy loss in this process. Hence, the energy gain provided by the ketones is actually less than the energy cost of making them, at least in theory.
This suggests that being in nutritional ketosis may require more overall system energy, while still increasing work potential. In other words, a person in nutritional ketosis may increase their overall energy expenditure, while at the same time increasing their muscular efficiency. In honor of Kevin, I refer to this as the Hall Paradox.
Parting shot
Ok, if you’re still reading this, give yourself a pat on the back. This was a bit of chemistry tour de force. Why did I do it? Well, frankly, I’m tired of reading so much nonsense on this topic. Everybody with a WordPress account (and countless people without) feels entitled to spew their opinions about ketosis without even the slightest clue of what they are talking about. As I said in part I of this series, there is no bumper sticker way to address this question, so to say ketosis is “good” or “bad” without getting into the details is as useful as a warm bucket of hamster vomit (unless you’re Daniel Tosh, in which case I bet you can find a great use for it).
Next time, I’ll try to back it out of the weeds and get to more clinically interesting stuff. But we had to do this and we’re better for it.
Chemistry by Marcin Wichary is licensed under CC by 2.0





Good afternoon, Peter.
I have mostly been in ketosis for the last year, ever since I decided to change my eating habits.
Now, I am an avid coffee-drinker. Although some people agree or disagree about the effects of caffeine on ketosis, lypolisis and insulin resistance, I was wondering about your perspective on this compound, and the effects on the latter states mentioned.
More importantly, does science point to mostly negative effects on caffeine, or positive?
Thank you very for your time and contribution in the field of scientific nutrition, and I am looking forward to part III.
P.S. I live in Montreal and I now have a wide field of knowledge in the subject after reading many articles by Taubes and yourself, to the point where I actually wrote a 15-page essay on the leading causes of obesity in the West, where I concluded that carbohydrates were mostly the cause, when combined with poor genetics. Essentially, I have enough confidence to go out and explain this metabolic state and its advantages, as well as the metabolic problems of a high carbohydrate diet. What can I do to contribute to the research and awareness?
Caffeine in most people probably increases sympathetic tone. This would drive up lipolysis (via HSL) and hepatic glucose output. Net result would probably raise glucose slightly.
Peter,
I have to thank you for this information. I am Type II diabetic and I have normal glucose. I have lost 50 lbs. I have an A1C last measured at 5.1. My trigs are 96. HDL 41. It has been over a year since my A1C was 8.6. Your information has helped me stick with it.
Keep going. It will take some time for others follow your lead. But you know the statistics. It will be worth it.
Amazing story, Ed, especially when the convention wisdom says the following: T2D is a chronic disease that only be managed with medical (re: drug) therapy.
Thank you so much for this series, Peter. As an undergraduate biology student I find this blog most interesting.
Your knowledge is much in coordinate with this reading I just came across how ketosis can facilitate brown fat concentrations! https://www.nyas.org/Publications/EBriefings/Detail.aspx?cid=f2c6ffae-5166-4478-a01d-261c6742d2c2
I would love to help out anyway I could with NuSI as an undergraduate, as I am a firm believer in the Alternative Hypothesis myself.
Thanks, Austin. Please direct any NuSI inquiries to NuSI.
Can somebody…anybody please answer this question for me. I found where someone else had asked this question, but it was never answered, and I have spent many unsuccessful days trying to find the answer online.
I want to do some tests (amending my diet) to determine what is keeping me from getting into NK. I have the monitor, I have the strips. WHAT I DON’T KNOW IS HOW SOON AFTER I EAT, WILL MY BLOOD KETONES BE AFFECTED?? PLEASE HELP ME =)
A 69 year old friend in England has terminal cancer. I read of a doctor who was given 3 months to live because of cancer. He went onto the LCHF diet and this got rid of all the cancer cells. My friend is now trying it and after a few weeks already feels she has more energy. However, she is loosing weight, which she can’t afford as she is already thin. How can she ensure she won’t loose? Then she has heard that this diet puts strain on the liver and that is where she has some tumours. I have not heard this. What comments or advice can anyone give please? It would be good if she could find a doctor who believes in NK.
Thanks for sharing so much interesting stuff. I run ultramarathons 50 miles up to 48 hrs and have a history of “bonking” after 10-15 hrs of running/jogging/walking. My stomach cramp, energy drops big time and within an hour it’s like running while having the flu. I’ve tried eating a lot, eating not so much and eating different stuff. Nothing has worked out so far. So a week ago, after reading about keto-adaption, I decided to go fat 70% protein 20% and carbs 10% approx.
First 2-3 days I could’ve killed for sugar but energy level was only slightly lowered (no running). After that my temper has stabilized back to normal and I’ve done some 1/2 hour slow runs. Energy level while running is still a bit lowered but in general on my base level.
Now, today (on day 5) I took a lab. test on ketone in urine which showed 3+. The manual at the lab (wife work there) said 3.9-8.7 mml/l some sites on internet say 3+ = 1.4-5.2 mml/l. In any case it’s a relatively high level. My question is, if this is a true positive, can you really get that result from 5 days of keto-diet unless you had a rather high baseline to begin with? If that is possible and I’ve gone from a “normal” level of 0-0.5 in this short time shouldn’t my subjective experience of change be a bit more obvious. As noted I don’t feel that much different in any aspect, physically or mentally.
For the record I’m 47yrs and diagnosed with adhd (treated w methylphenidate) and my staple food has always been much of everything, especially fast carbs.
Anyways, I’m very surprised by the test result and eager to find out if I am (a) an anorectic alkoholic with diabetes, (b) a potential fat-burning machine par excellance or (c) something completely different.
I’ll figure out the last Q but would appreciate some input regarding the seemingly high increase in ketone after only 5 days. Some general clues or tips on how to go from here would do. I’m fully aware that a proper diagnose and treatment plan, so to speak, is way beyond the possible.
Niklas, See if you can gather some objective data. For example, take “race pace” and hold that on a treadmill for 60 min (after a 60 min warmup) with VO2 and VCO2 apparatus to measure your RQ. This will tell you how you access your energy stores.
Gotta love the generalized applicability of thermodynamics!
Thank you for the great blog. I have learned a lot and started on quite a transformation. I am intermittent fasting 20 hours a day and refeading with a healthy very low carb diet. After 8 weeks I am down 25 pounds but my lipid panel has gotten terrible. Have cut out all sugar and processed foods now my MD wants to put me on meds for high triglycerides and wants me to stop the low carb lifestyle. What are your thoughts?
Impossible to say, but I bet your TG is especially impacted by the timing of the draw given your huge meal the night before.
Hi Peter,
I recently had a standard lipid done after a little more than 20 hours of fasting which followed a pretty big meal. All numbers and ratios were good but I dad think about the timing and size of my last meal before the draw. I would have thought that 20 hours of fasting would be enough.
How would you recommend eating/fasting prior to a standard lipid test to get the most reliable results?
Thank you,
Hemming
Whatever you believe is representative of your eating. Snapshot tests (e.g., fasting TG) are probably less helpful than AUC tests, but most non-snapshot testing is impractical. Lipids are not impacted much by recent meal. Glucose, insulin, TG are more sensitive.
I’m living the ketogenic life style, but I keep reading that high fat diets are highly correlated to prostate cancer! I’m in the 20% tile group and worry that if it’s true I’ll lose the battle trying to win the war. Ansel Keys was fooled his statistics… any thoughts?
Hi Peter,
Just a request for the subsequent parts of this series (if you still plan to do them). I would love to see you include the different possible culprits for people who have difficulty adapting to keto (despite plenty of time for the adaptation period, sodium, low enough protein).
I recently cut carbs well below 50, adding in broth religiously and keeping protein low, and maintained that for 8 weeks with no cheating and still had exceedingly low energy levels. Is it possible that, for some people, the added metabolic cost of producing ketones is enough to make them feel tired indefinitely on this eating protocol?
Also, we are hearing more and more about the dangers of low carb (thyroid function, stress on adrenals, lack of starches for beneficial gut bacteria, etc.). I would love to hear whether you think the evidence supporting these warnings is well founded.
The reason I am so interested in a keto diet is that when I am not eating very low carb I tend to binge on carbs. For some reason I seem completely unable to eat “moderate carb”, as many are now recommending. I have been trying the moderate carb, “safe starch” approach for weeks now, but I always end up bingeing on very high carb stuff. Yet when I was eating keto for 8 weeks I never cheated at all and did not crave carbs (I stopped because my general energy was so low, and all the low-carb-will-blow-out-your adrenal warnings began to scare me.)
Probably worth a quick post on keto troubleshooting at some point.
This isn’t mentioned in your article (nor have I seen it mentioned elsewhere), but doesn’t the fact that someone in ketosis expels a bunch of energetic acetone indicate that ketosis wastes energy (ie, that you use more calories in ketosis)?
The amount and energy content of acetone in breath (and AcAc in urine) is between small and negligible.
I can not believe I found other people in this world with similar viewpoints on nutrition. I have been in ketosis since 2001 after reading an article online about the Atkins diet. The journey into a ketogenic world started out as an experiment to see if a person could shave body fat without restricting calories enough to catabolize muscle (I had bodybuilder friends who wanted to get ripped and I thought Atkins might be worth trying so I tried it for them). So the Atkins experiment morphed into cutting out sugar and from there to no more than 20 gms of carbs a day if possible. That morphed into only organic foods. From then on it’s been fine tuning every so often such reading everything I could find concerning fats and oils (nutritiondata.com is great about going into in-depth detail about food composition) and experimenting to see what ratios worked best for me.
I’ve never really came across anything (but I’ve not looked that closely) about people being on a ketogenic diet for decades or life. My blood work always comes back great but I’m approaching my mid 40’s now and I’m a little concerned about how this may have affected my body chemistry or certain gene expressions over a long period of time. I wonder things like: am I getting plaque in my arteries, are my telomeres shortening quicker than someone with a more even carb/protein/fat ratio? Stuff like that. Any thoughts about long term effects of ketogenic diet?
I also personally believe that there is a difference in mood, concentration and how easy those are to control when relying on ketones for fuel. That’s only a personal opinion. Just found this blog today and found it interesting. I knew there were groups of people out there who ate very low amounts of carbohydrates but didn’t know there was anyone dissecting it in such detail. Great reads!
Would also be very curious about any information on best fats (fatty acids) to eat in terms of their ratios (ie mono, poly, saturated). Not really in terms of those ratios for weight control but rather for optimal long term health.
Dr. Attia,
I bought ketone urine test strips and my readings are 5 to 10 mg/dl. Does that correlate with my daily carb intake of 50 to 100 grams?
Thanks
Urine strips are of little (no?) value, in my opinion.
Thanks for this blog. Ketogenic eating has been a life changer for me and it’s good to get some more of the science behind it.
I too would be interested in knowing more about long-term effects of keto, though my current understanding is that there aren’t all that many longevity studies on this… anything you know of would be helpful.
Also, I have a friend who is very underweight and has struggled for her whole life to keep her weight up. She is interested in ketogenic diets because she has extreme blood sugar crashes regularly (she does not have any indications of allergies or diabetes). How would this way of eating affect a person like her? Would she have to do anything differently from those who lose or maintain weight on it?
Not really sure.
Phinney LE book on performance has a section where he discusses how exercise may actually NOT help speed metabolism or expenditure after exercise. He states that 4 well controlled ward studies evidence that and no good studies refute it. Just wanted to get ur take on that.
Correct, no reason to believe ketosis will boost anaerobic capacity. The question is how long does it take to adapt to the point where it’s not hurting anaerobic performance? In me, the answer was 18 months.
Peter, have you seen any data showing how many ketones can be produced from different types of fats: monounsaturated, polyunsaturated (omega3 and omega6), and saturated fat (including examples from each of the four fatty acid groups (e.g., lauric acid, stearic, etc)).
I have started my first experiments with ketosis, and for whatever reason I had problems entering ketosis rapidly on monounsaturated fats. Mixing many fats over four hours got me there. Not sure if I have a timing issue, absorption problem (quite probable in my case), or if different fats do in fact stimulate different levels of ketone production.
This topic is very relevant to what you focus on in this blog, and I think it would be worth of a separate blog post, assuming there is any literature of note to summarize.
Yes, but it probably varies by person and metabolic and hormonal milieu. Medium chain triglycerides of SFA, are generally most potent, with C8 (caprylic) being potentially the most ketogenic.
Peter, caprylic would basically be MCT Oil? I was using a large quantity of MCT Oil, but it makes me feel uneasy. It may be too thermogenic. As you say, there may be a lot of unique personal biology involved.
If anyone else has studied this topic, it would be interesting to see this all quantified? In other words, for X gram ingested of different fats, how many ketones are produced at the peak level? It should be easy to approximate an answer to this by eating just one oil for four hours, starting from a ketone level of <= 0.2 mmol/L and then measure every hour until the peak is achieved.
MCT oil is to pants what caprylic is to jeans.
Started to do a research search on my question, and wow immediately finding very interesting data. Look at this study comparing ketones between saturated and polyunsatured diets:
https://www.ncbi.nlm.nih.gov/pubmed/15070924
The polyunsaturated diet produced 2.7 TIMES as many ketones compared to saturated fat. Obviously most of us don’t want poly fats for other reasons, but this study is enough to make me think my intuition to study this question will produce fruit. I will report back after I have a summary of more studies.
Hey Peter,
Whatever happened to the rest of this series. I read every comment, and a lot of your responses refer to the upcoming parts of this series, but I can’t seem to find them anywhere.
Still in my head.
Hello Peter,
Would you advise the American professional cycling team of Novo Nordisk (All cyclists with type 1 diabetes) to go ketogenic? Does the KD also reverse and improve on this type of diabetes?
Not necessarily. Definitely reduced GI and sugar intake, but this would actually be the perfect group for superstartch, just as it was developed for kids with GSD.
T1D does not “resolve” with diet, but diet can reduced greatly the need for insulin and as such the morbidity of the disease.
Hi Dr Attia,
I use short term protein sparing modified fasts as a tool to reduce my “cutting” cycles on a strength athlete training protocol. It has worked really well with very little hunger. I have heard criticism to the effect that since carbohydrates are “needed” for gluconeogenesis that removing carbs is ultimately not optimal because some carbs are “needed” to efficiently convert fat into energy.
While I don’t doubt the chemistry, my suspicion is that people are overestimating how many grabs of carbohydrates are needed to run the process and overestimating how effectively you can remove ALL carbs from your diet even if you wanted to.
Could you please provide a rough estimate on how many grams of carbohydrates (or C6 H12 O6) are needed to burn a pound of fat? It would be a helpful number to help put things in context. My feeling is that even on a PSMF, the broccoli and spinach leaves I’m eating is low enough in carbs to keep my in/around ketosis but high enough to provide the raw material to run the complex reactions above (which in trusth can’t really follow).
-M
Technically one does not need to ingest 1 g of CHO to mobilize and oxidize fat. Otherwise starvation would kill us.
I’ve the ketogenic diet, but blood tests cost me Htrigtzridim and the LDL level is very high.
I do CrossFit athlete loads and power, maybe I’m missing salts, magnesium, etc.