Check out more content with expert, Matt Kaeberlein, Ph.D.:
- (Aug 20, 2018) Rapamycin and dogs — man’s best friends? — living longer, healthier lives and turning back the clock on aging and age-related diseases
- (Sep 13, 2021) The biology of aging, rapamycin, and other interventions that target the aging process
- (May 16, 2022) AMA #35: “Anti-Aging” Drugs — NAD+, metformin, & rapamycin
Dr. Matt Kaeberlein is a globally recognized expert on the biology of aging and recurring on The Drive. In this episode, Matt explains his research findings on nutrition as it relates to aging and longevity, including the results from his recent review article in Science. From there, he and Peter dive deep into the literature on calorie restriction (CR), explaining the nuance, benefits for lifespan and healthspan, and potential downsides of CR. He discusses the epigenetic changes that occur with age and potential benefits and downsides of epigenetic reprogramming, often viewed as a panacea for reversing aging. Matt also explains the impact of dietary protein on aging, including the interesting dichotomy around how protein, a critical macronutrient, and rapamycin, a geroprotective molecule, have opposite effects on mTOR. Additionally, he talks about low-protein vs. high-protein diets and their effects on muscle mass and mortality, as well as the impact of IGF-1 signaling and growth hormone on lifespan.
Subscribe on: APPLE PODCASTS | RSS | GOOGLE | OVERCAST | STITCHER
We discuss:
- Challenges with understanding the effects of nutrition and studying interventions for aging [3:30];
- How Peter’s and Matt’s convictions on nutrition and thoughts optimal health have evolved [8:15];
- Calorie restriction for improving lifespan in animal models [16:15];
- Utility of epigenetic clocks and possibility of epigenetic reprogramming [22:00];
- Mutations and changes to the epigenome with aging [31:45];
- Epigenetic reprogramming: potential benefits and downsides and whether it can work in every organ/tissue [35:15];
- First potential applications of anti-aging therapies and tips for aging well [43:00];
- Impact of calorie restriction on the immune system, muscle mass, and strength [47:00];
- Insights from famous calorie restriction studies in rhesus macaques [55:00];
- An evolutionary perspective of the human diet [1:03:45];
- Anti-aging diets: Separating fact from fiction—Matt’s 2021 review in Science [1:12:30];
- Mouse models of time-restricted feeding in the context of calorie restriction [1:19:30];
- Nutritional interventions that consistently impact lifespan in mice, and concerns around efficacy in humans [1:27:00];
- Differing impact of calorie restriction when started later in life [1:31:00];
- Lifespan extension with rapamycin in older mice [1:37:15];
- Relationship between protein intake and aging, and mouse studies showing protein restriction can extend lifespan [1:43:30];
- Impact of protein intake on mTOR, and why inhibition of mTOR doesn’t cause muscle loss [1:50:45];
- Low-protein vs. high-protein diets and their effects on muscle mass, mortality, and more [1:55:30];
- The impact of IGF-1 signaling and growth hormone on lifespan [2:06:30];
- Parting thoughts on the contribution of nutrition to healthspan and lifespan [2:19:45];
- More.
Challenges with understanding the effects of nutrition and studying interventions for aging [3:30]
- Peter remarks, “I don’t think a week goes by that we aren’t exchanging an email about some aspect of the relationship or the interspace between nutrition and longevity.”
Questions‒
- Does that speak to our ignorance?
- Does that speak to the ubiquity of such content?
- What does that say about us?”
- Matt’s take, “Having been in the aging field for a long time, I certainly recognize how complicated that biology is. And I think the biology of nutrition is equally complicated. And when you get at the interface of those two it’s really hard, I think, sometimes to draw definitive conclusions.”
- He also thinks this will be a little bit of a theme‒ there are many things we don’t understand yet about optimal nutrition and how that intersects with optimal health span
- Peter and Matt have spent a lot of time on the podcast speaking about molecules that may protect against aging: rapamycin, and more recently‒ NMN, NAD, and Metformin
- #10 – Matt Kaeberlein, Ph.D.: rapamycin and dogs — man’s best friends? — living longer, healthier lives and turning back the clock on aging and age-related diseases
- #175 – Matt Kaeberlein, Ph.D.: The biology of aging, rapamycin, and other interventions that target the aging process
- #207 – AMA #35: “Anti-Aging” Drugs — NAD+, metformin, & rapamycin
- It’s almost easier to ask these questions from the standpoint of geroprotective molecules because the intervention is much cleaner
- Just yesterday, Peter, Matt and David Sabatini had a back and forth about rapamycin‒ timing of the dose, frequency within the dosing schedule, the dose itself
- Even with a drug this can be complicated
- The benefits of a drug are still trying to be pieced together, imagine putting this together for the topic of food/ nutrition
- Matt adds, “The big piece that gets lost with the animal models on top of all that complexity is the environment”
- In animal studies, the mice are kept in a well controlled environment, relatively pathogen free, and they live in that same environment their entire life
- Compare that to the human experience where our environment is extremely complicated, and it changes throughout life
- Also, the epidemiological studies on optimal nutrition are from 20, 30, 40 years ago
- The average human environment is very different today than it was when those studies were done
- How does that potentially change the interaction between nutrition and health outcomes?
Difficulties of studying interventions for aging
- People age very slowly
- People in their 70s today were in their 30s 30 years ago, and that environment was different than today’s environment
“I think the key is to recognize that limitation and be potentially even more careful about assuming causation from correlation over many decades”‒ Matt Kaeberlein
How Peter’s and Matt’s convictions on nutrition and thoughts optimal health have evolved [8:15]
How Peter’s convictions on nutrition have changed over the past decade:
- His convictions have become looser as time goes on
- He views fewer and fewer things with certainty
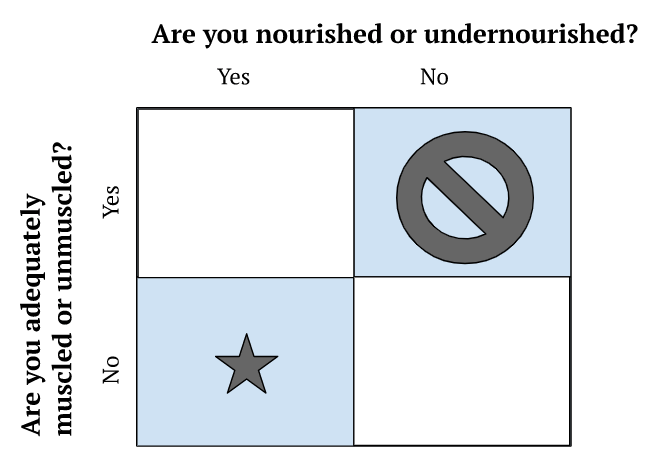
- Clinically, Peter views nutrition from a very simple 2×2 framework
Figure 1. 2×2 clinical framework for viewing nutrition.
- People are not distributed equally in these buckets
- There are not many people who are adequately muscled and undernourished
- You can’t sort someone into a bucket by looking at them
- You need to do some functional testing and look at their biomarkers
- This might include a DEXA scan
- It’s important to sort people into a bucket to answer the questions‒
- Do you need an energy deficit?
- Do you need an energy surplus?
- What’s your protein intake need to be to achieve that in combination with your calorie needs?
- By far, the hardest to treat is the “over nutritioned, under muscled”
- Unfortunately that’s a very common phenotype
- Matt agrees with this approach and adds, “There’s no one size fits all… and it’s is an ongoing learning process”
- It’s really important that we be willing to change our beliefs about nutrition and other aspects of health as more data comes in
Problems with the changing advice of nutritionists
{end of show notes preview}

Matt Kaeberlein, Ph.D.,
Matt Kaeberlein, Ph.D., is a Professor of Pathology, Adjunct Professor of Genome Sciences, and Adjunct Professor of Oral Health Sciences at the University of Washington. His research interests are focused on basic mechanisms of aging in order to facilitate translational interventions that promote healthspan and improve quality of life. He has published nearly 200 papers in top scientific journals and has been recognized by several prestigious awards, including a Breakthroughs in Gerontology Award, an Alzheimer’s Association Young Investigator Award, an Ellison Medical Foundation New Scholar in Aging Award, a Murdock Trust Award, a Pioneer in Aging Award, and the Vincent Cristofalo Rising Star in Aging Research. His contributions have also been recognized with Fellow status in the American Association for the Advancement of Science, the American Aging Association, and the Gerontological Society of America. Dr. Kaeberlein is a past President of the American Aging Association and has served on their Executive Committee and Board of Directors since 2012. He has also served as a member of the Board of Directors for the Federation of American Societies for Experimental Biology and is currently the Chair of the Biological Sciences Section of the Gerontological Society of America. Dr. Kaeberlein serves on the editorial boards for several journals, including Science and eLife. Dr. Kaeberlein’s scientific discoveries have generated substantial public interest, with featured stories in major media outlets including appearing on the front page of the New York Times, the Today Show, CNN, the UK Telegraph, Popular Science, Time Magazine, Scientific American, NPR, USA Today, National Geographic, and many others. In addition to his primary appointments, Dr. Kaeberlein is the founding Director of the Healthy Aging and Longevity Research Institute at the University of Washington, the co-Director of the University of Washington Nathan Shock Center of Excellence in the Basic Biology of Aging, and founder and co-Director of the Dog Aging Project. [kaeberleinlab.org]
Facebook: Matt Kaeberlein
Twitter: @mkaeberlein





This is a great podcast!! I’m 69 years old male currently recovering from a low grade noninvasive bladder cancer. I had a kidney transplant 37 years ago last week.
Once I have fully recovered from my last surgery about late November, I want to become a member and follow a fittest centenarian Olympic program.
I’m looking forward to the training.
Early on in the episode Dr. Kaeberlein mentions that we need a study showing how activation of the Yamanaka factors in old mice would be the next step in the knowledge gap to fill (with regards to genomically controlled de-aging), and mentioned the literature hasn’t quite gotten to that experiment yet.
Not So! This exact work has been performed in the the Belmonte lab at the Salk (one of his last papers here before moving on to Altos). Essentially, they engineered the 4 classical Yamanaka factors (OSKM), and critically, put them under control of a Dox inducible promoter, and engineered this into mouse embryos (so this control scheme is in every cell of the mouse). In other words, the factors could be activated/deactivated by including doxycycline in the mouse chow.
Young mice were “resistant” to aging, and old mice were de-aged (transcriptomically speaking), to varying degrees, but overall it works ( . . . in mouse).
https://www.nature.com/articles/s43587-022-00183-2
I liked this. We want a simple recommendation on what to eat but as always there are so many confounding factors. But, putting all the best guesses of what we think we know can lead to good results. So I:
* eat a variety of fresh vegetables, from my garden when possible, lean animal protein usually from my local farmers market. Nuts and berries, too.
* resistance train regularly and have for some time.
* take cod liver oil and test my omega-3’s at Omega Quant (due of this podcast).
* wear a glucose monitor keeping spikes to a minimum.
* meditate.
* cold water exposure.
* use breathing techniques.
As a 70 year old male, I am a standout in my age group. Thanks for the great info.
Valued member, Bob
Obviously I love The Drive, and just about every episode is worth listening to. But this one, while again fascinating, was a bit of a bait-and-switch. When most of us think of “nutrition,” it’s not rapamycin or IF or FMD or TRF, it’s what we actually *are* eating. Aside from the discussion of protein amounts (what kind of protein, does it matter?), and not overeating, there was almost nothing in this podcast related to “nutrition” in the conventional sense: vitamins, minerals, phytochemicals, probiotics, prebiotics, antioxidants, fiber, glycemic load–and how you get the benefit of these things from eating FOOD. What about a having a guest that likes to talk about this? Luigi Fontana is one suggestion. Thanks very much and keep up the good work.
Sticking to the area of nutrition you’re more interested in, protein, do you see a difference between animal vs plant protein?
As you say, it’s hard to draw concrete conclusions from nutritional studies, but is there enough evidence to say that consuming plant protein reduces all cause mortality compared to animal protein? I’m looking at the papers that Dr Michael Greger sites – https://nutritionfacts.org/video/animal-protein-vs-plant-based-protein/
We know you suggest we probably all need to eat more protein, and maybe you think it’s unrealistic to hit these targets without animal protein? Or do you think these studies are flawed?
Sorry if you’ve covered this in a past podcast – I’ve searched the back catalog and can’t find an answer.
regarding Peter’s comments/experimentation with 1gm protein/lb bodyweight, I’ve tried to do the same with combination of food, whey, and EAAs. I’m 63, an age-group athlete with resistance training, and easily anabolic WRT muscle. When I get to over about 100 gms protein, especially with EAAs, my sweat has strong ammonia smell, which I have read is a sign that my body isn’t processing protein… Is this true? is that a sign of too much protein? Is there a blood, etc test? … could just be the brand of EAAs — pure bulk powder, which doesn’t smell/taste good before it goes in!
Has anyone done a test of the difference in bioavailability of sirolimus from Dr. Reddy’s triangular pills and the sirolimus made by Glenmark? Although I have no scientific proof, after taking both brands over a period of several months, I far prefer Glenmark. The pills are much smaller and easier to swallow and I don’t understand why for the same 1mg dose of rapamycin one would need a much larger triangular pill. Lastly, I had an itchy skin rash from Dr. Reddy’s pills but zero side effects from Glenmark. It would be nice to know more about it.