One night, as I alluded to in this post, Tim and I were having dinner and the topic of cancer came up. Personally and professionally I have a great interest in cancer, so when Tim asked if I could write something about cancer that was: (i) interesting to a broad audience, (ii) not technically over the top, (iii) not my typical 5,000 word dissertation, (iv) yet nuanced enough for his readers, I agreed to give it a shot, in about 1,000 words. (The content of this blog went up on Tim’s blog last week, but I’ve reproduced it here, less Tim’s commentary.)
Semantics and basics
Before jumping into this topic I want to be sure all readers — regardless of background — have a pretty good understanding of the ‘basics’ about cancer and metabolism. In an effort to do this efficiently, I’ll list concepts here, such that folks can skip them if they want to, or refer back as necessary. This way, I don’t need to disrupt the ‘story’ with constant definitions. (Yes, I realize this is sort of cheating on my 1,000 word promise.)
Cancer – a collection of cells in our bodies that grow at roughly normal speeds, but that do not respond appropriately to cell signaling. In other words, while a collection of ‘normal’ cells will grow and stop growing in response to appropriate messages from hormones and signals, cancer cells have lost this property. Contrary to popular misconception, cancers cells do not grow especially fast relative to non-cancer cells. The problem is they don’t ‘know’ when to stop growing.
Metabolism – the process of converting the stored energy in food (chemical energy contained mostly within the bonds of carbon and hydrogen atoms) into usable energy for the body to carry out essential and non-essential work (e.g., ion transport, muscle contraction).
ATP – adenosine triphosphate, the ‘currency’ of energy used by the body. As its name suggests, this molecule has three (tri) phosphates. Energy is liberated for use when the body converts ATP to ADP (adenosine diphosphate), by cutting off one of the phosphate ions in exchange for energy.
Glucose – a very simple sugar which many carbohydrates ultimately get broken down into via digestion; glucose is a ring of 6-carbon molecules and has the potential to deliver a lot, or a little, ATP, depending on how it is metabolized.
Fatty acid – the breakdown product of fats (either those stored in the body or those ingested directly) which can be of various lengths (number of joined carbon atoms) and structures (doubled bonds between the carbon atoms or single bonds).
Aerobic metabolism – the process of extracting ATP from glucose or fatty acids when the demand for ATP is not too great, which permits the process to take place with sufficient oxygen in the cell. This process is highly efficient and generates a lot of ATP (about 36 units, for example, from one molecule of glucose) and easy to manage waste products (oxygen and carbon dioxide).
The process of turning glucose and fatty acid into lots of ATP using oxygen is called ‘oxidative phosphorylation.’
Anaerobic metabolism – the process of extracting ATP from glucose (but not fatty acids) when the demand for ATP is so great that the body cannot deliver oxygen to cells quickly enough to accommodate the more efficient aerobic pathway. The good news is that we can do this (otherwise a brief sprint, or very difficult exertion would be impossible). The bad news is this process generates much less ATP per carbon molecule (about 4 units of ATP per molecule of glucose), and it generates lactate, which is accompanied by hydrogen ions. (Contrary to popular belief, it’s the latter that causes the burning in your muscles when you ask your body to do something very demanding, not the former).
Mitochondria – the part of the cell where aerobic metabolism takes place. Think of a cell as a town and the mitochondria as the factory that converts the stored energy into usable energy. If food is natural gas, and usable energy is electricity, the mitochondria are the power plants. But remember, mitochondria can only work when they have enough oxygen to process glucose or fatty acids. If they don’t, the folks outside of the factory have to make due with suboptimally broken down glucose and suboptimal byproducts.
DNA – deoxyribonucleic acid, to be exact, is the so-called “building block” of life. DNA is a collection of 4 subunits (called nucleotides) that, when strung together, create a code. Think of nucleotides like letters of the alphabet. The letters can be rearranged to form words, and words can be strung together to make sentences.
Gene – if nucleotides are the letters of the alphabet, and DNA is the words and sentences, genes are the books – a collection of words strung together to tell a story. Genes tell our body what to build and how to build it, among other things. In recent years, scientists have come to identify all human genes, though we still have very little idea what most genes ‘code’ for. It’s sort of like saying we’ve read all of War and Peace, but we don’t yet understand most of it.
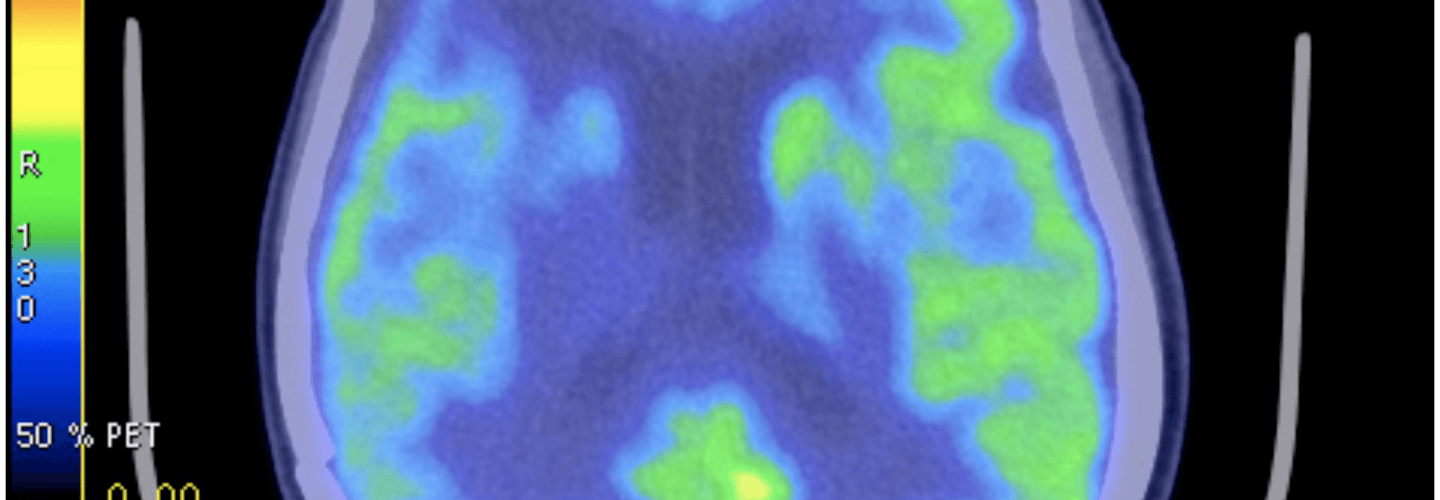
FDG-PET – a type of ‘functional’ radiographic study, often called a ‘pet scan’ for short, used to detect cancer in patients with a suspected tumor burden (this test can’t effectively detect small amounts of cancer and only works for ‘established’ cancers). F18 is substituted for -OH on glucose molecules, making something called 2-fluoro-2-deoxy-D-glucose (FDG), an analog of glucose. This molecule is detectable by PET scanners (because of the F18) and shows which parts of the body are most preferentially using glucose.
Phosphoinositide 3-kinase – commonly called PI3K (pronounced “pee-eye-three-kay”), is an enzyme (technically, a family of enzymes) involved in cell growth and proliferation. Not surprisingly, these enzymes play an important role in cancer growth and survival, and cancer cells often have mutations in the gene encoding PI3K, which render PI3K even more active. PI3Ks are very important in insulin signaling, which may in part explain their role in cancer growth, as you’ll see below.
The story (in about 1,000 words, as promised)
In 1924 a scientist named Otto Warburg happened upon a counterintuitive finding. Cancer cells, even in the presence of sufficient oxygen, underwent a type of metabolism cells reserved for rapid energy demand – anaerobic metabolism. In fact, even when cancer cells were given additional oxygen, they still almost uniformly defaulted into using only glucose to make ATP via the anaerobic pathway. This is counterintuitive because this way of making ATP is typically a last resort for cells, not a default, due to the very poor yield of ATP.
This observation begs a logical question? Do cancer cells do this because it’s all they can do? Or do they deliberately ‘choose’ to do this? I’m not sure the answer is entirely clear or even required to answer the macro question I’ve posed in this post. However, being curious people we like answers, right?
The first place to look is at the mitochondria of the cancer cells. Though not uniformly the case, most cancers do indeed appear to have defects in their mitochondria that prevent them from carrying out oxidative phosphorylation.
Explanation 1
Cancer cells, like any cells undergoing constant proliferation (recall: cancer cells don’t stop proliferating when told to do so), may be optimizing for something other than energy generation. They may be optimizing for abundant access to cellular building blocks necessary to support near-endless growth. In this scenario, a cancer would prefer to rapidly shuttle glucose through itself. In the process, it generates the energy it needs, but more importantly, it gains access to lots of carbon, hydrogen, and oxygen atoms (from the breakdown of glucose). The atoms serve as the necessary input to the rate-limiting step of their survival — growth. The selection of cancer cells is based on this ability to preferentially grow by accessing as much cellular substrate as possible.
Explanation 2
Cells become cancerous because they undergo some form of genetic insult. This insult – damage to their DNA – has been shown to result in the turning off of some genes (those that suppress tumor growth) and/or the activation of other genes (those that promote cell growth unresponsive to normal cell-signaling). Among other things, this damage to their DNA also damages their mitochondria, rendering cancer cells unable to carry out oxidative phosphorylation. So, to survive they must undergo anaerobic metabolism to make ATP.
Whichever of these is more accurate, the end result appears the same – cancer cells almost exclusively utilize glucose to make ATP without the use of their mitochondria. A detailed discussion of which explanation is better is beyond the scope of my word allotment, and it’s not really the point I want to make. The point is, cancer cells have a metabolic quirk. Regardless of how much oxygen and fatty acid they have access to, they preferentially use glucose to make ATP, and they do it without their mitochondria and oxygen.
So, can this be exploited to treat or even prevent cancer?
One way this quirk has been exploited for many years is in medical imaging. FDG-PET scans are a useful tool for non-invasively detecting cancer in people. By exploiting the obligate glucose consumption of cancer cells, the FDG-PET scan is a powerful way to locate cancer (see figure).
You can probably tell where I’m leading you. What happens if we reduce the amount of glucose in the body? Could such an intervention ‘starve’ cancer cells? An insight into this came relatively recently from an unlikely place – the study of patients with type 2 diabetes.
In the past few years, three retrospective studies of patients taking a drug called metformin have shown that diabetic patients who take metformin, even when adjusted for other factors such as body weight and other medications, appear to get less cancer. And when they do get cancer, they appear to survive longer. Why? The answer may lie in what metformin does. Metformin does many things, to be clear, but chief among them is activating an enzyme called AMP kinase, which is important in suppressing the production of glucose in the liver (the liver manufactures glucose from protein and glycerol and releases it to the rest of the body). This drug is used in patients with diabetes to reduce glucose levels and thereby reduce insulin requirement.
So, the patients taking metformin may have better cancer outcomes because their glucose levels were lower, or because such patients needed less insulin. Insulin and insulin-like growth factor (IGF-1) also appear to play an integral role in cancer growth as recently demonstrated by the observation that people with defective IGF-1 receptors appear immune to cancer. Or, it may be that activation of AMP kinase in cancer cells harms them in some other way. We don’t actually know why, but we do know that where there is smoke there is often fire. And the ‘smoke’ in this case is that a relatively innocuous drug that alters glucose levels in the body appears to interfere with cancer.
This may also explain why most animal models show that caloric restriction improves cancer outcomes. Though historically, this observation has been interpreted through the lens of less ‘food’ for cancer. A more likely explanation is that caloric restriction is often synonymous with glucose reduction, and it may be the glucose restriction per se that is keeping the cancer at bay.
Fortunately this paradigm shift in oncology – exploiting the metabolic abnormality of cancer cells – is gaining traction, and doing so with many leaders in the field.
Over a dozen clinical trials are underway right now investigating this strategy in the cancers that appear most sensitive to this metabolic effect – breast, endometrial, cervical, prostate, pancreatic, colon, and others. Some of these trials are simply trying to reproduce the metformin effect in a prospective, blinded fashion. Other trials are looking at sophisticated ways to target cancer by exploiting this metabolic abnormality, such as targeting PI3K directly.
To date, no studies in humans are evaluating the therapeutic efficacy of glucose and/or insulin reduction via diet, though I suspect that will change in the coming year or two, pending outcomes of the metformin trials.
Last point (beyond my 1,000 word allotment)
Check out this blast from the past! Gary Taubes, who is currently working hard on his next book, came across the article the other day from 1887.
Influences
I’ve been absurdly blessed to study this topic at the feet of legends, and to be crystal clear, not one thought represented here is original work emanating from my brain. I’m simply trying to reconstruct the story and make it more accessible to a broader audience. Though I trained in oncology, my research at NIH/NCI focused on the role of the immune system in combating cancer. My education in the metabolism of cancer has been formed by the writings of those below, and from frequent discussions with a subset of them who have been more than generous with their time, especially Lewis Cantley (who led the team that discovered PI3K) and Dominic D’Agostino.
- Otto Warburg
- Lewis Cantley
- Dominic D’Agostino
- Craig Thompson
- Thomas Seyfried
- Eugene Fine
- Richard Feinman (not to be confused with Richard Feynman)
- Rainer Klement
- Reuben Shaw
- Matthew Vander Heiden
- Valter Longo
Further reading
I do plan to continue exploring this topic, but for those of you who want to know more right now and/or for those of you with an appetite for depth, I recommend the following articles, some technical, some not, but all worth the time to read. This is the short list:
- Relatively non-technical review article on the Warburg Effect written by Vander Heiden, Thompson, and Cantley
- Science piece written about cancer (for non-technical audience) by Gary Taubes
- Non-technical talk by Craig Thompson
- Detailed review article by Tom Seyfried
- Review article on the role of carb restriction in the treatment and prevention of cancer
- Talk given by author of above paper for those who prefer video
- Moderately technical review article by Shaw and Cantley
- Clinical paper on the role of metformin in breast cancer by Ana Gonzalez-Angulo
- Mouse study by Dom D’Agostino’s group examining role of ketogenic diet and hyperbaric oxygen on a very aggressive tumor model
- Mechanistic study by Feinman and Fine assessing means by which acetoacetate (a ketone body) suppresses tumor growth in human cancer cell lines
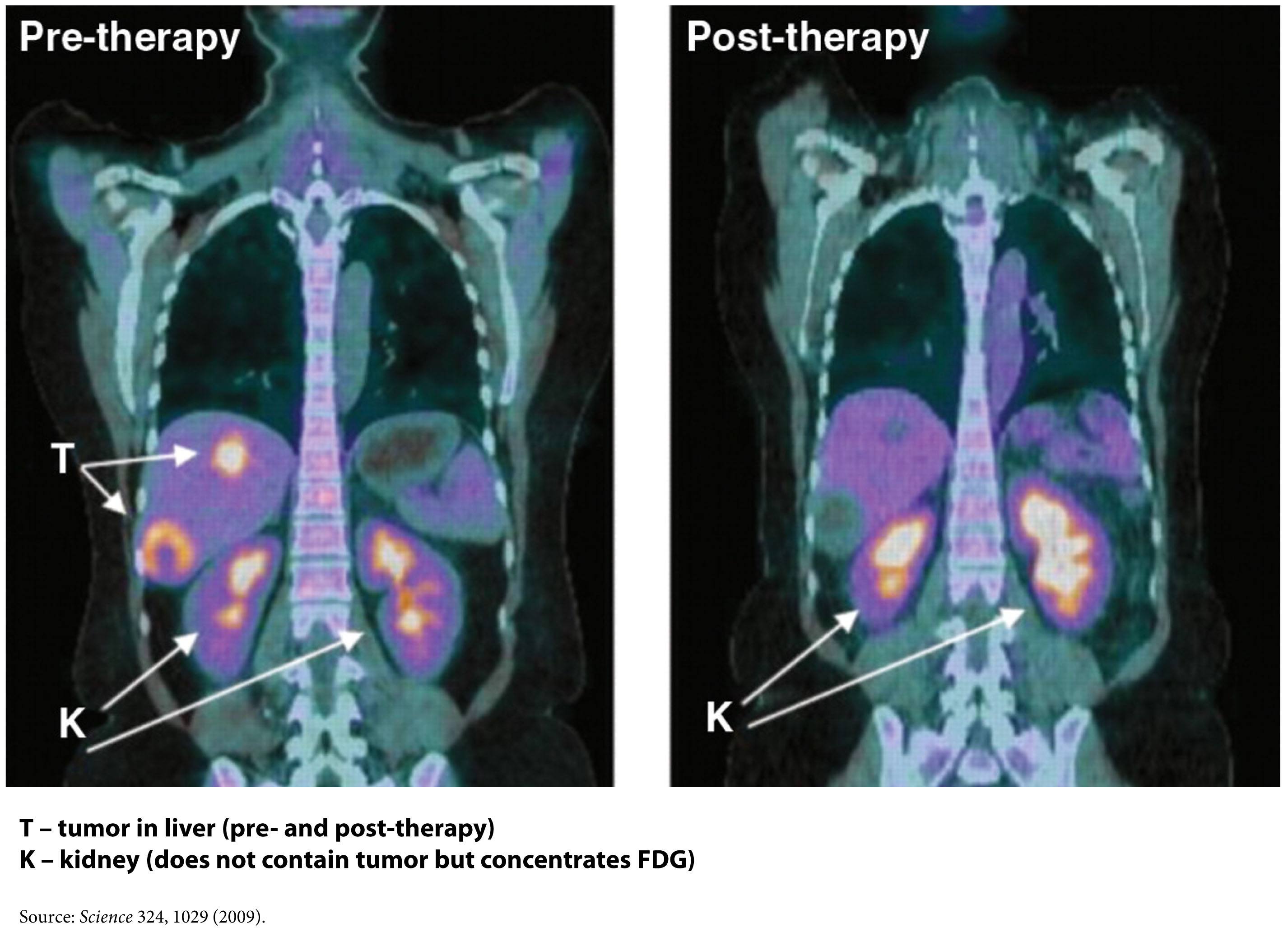
Figure of FDG-PET imaging showing no evidence of recurrent tumor after standard care treatment including a water-only fast and a ketogenic diet by Zuccoli et al., 2009 is licensed under CC by 2.0





“Dr. Freund, who is unknown as an authority, has advanced a theory wholly inconsistent with the results of years of scientific research.” Why are our scientists often so dismissive seeing that a lot of what was thought of as scientific fact have turned out to be false?
Thanks so much for the layman’s lesson in cancer metabolism. This will be great info for those in my family unfortunately currently struggling with type 2 & recurrent breast cancer.
The idea of “starving” the cancer by not eating carbohydrates doesn’t really add up for me very well. People on low carb have only slightly lower glucose concentrations in the bloodstream. There should be plenty to feed hungry cancer cells.
But, as I think you alluded to, what if the low carb diet ALSO causes changes in the hormone milieu that somehow does not allow the cancer cells to import glucose? Now THAT seems reasonable to me. Do cancer cells need insulin to import glucose?
Well it’s more nuanced than plasma glucose, as you allude to. On the plasma side, it’s probably more about the ratio of BHB to glucose — the closer to 1 (in mM), the better. For example, a BHB of 4 mM and a glucose of 75 mg/dL (about 4 mM) is a VERY different physiologic state that a BHB of 0 and a glucose of 85-100 mg/dL. Secondly, the role of insulin and IGF are much different in these scenarios. Same for other tumor growth factors.
Peter:
What a coincidence, since we just reconnected a few days and this happened in the interim.
https://freetheanimal.com/2014/02/medical-insurance-helping.html
In the meantime, I’ve been sent this:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3312698/
Then this:
https://www.damninteresting.com/coley’s-cancer-killing-concoction/
You want to think it’s tin-foil-hat but, I and a collaborator have been months into research and writing a book on the gut biome and I have come to believe that the right live bacteria in the right place are your targeted chemo therapists. Perhaps it’s more likely that someone in like 1890 might use the only thing available. From that last link:
“The records showed that after the wound became infected with a commonplace bacterium, Streptococcus pyogenes, the patient went through several bouts of fever. With each attack of fever the tumour shrank until eventually it disappeared entirely, leaving only a large scar under the left ear. Coley surmised that the infection had stimulated the German’s immune system– as evidenced by the repeated fevers– and that it was this immune response that had caused the eradication of the cancer.
“The story so convinced Coley that he– perhaps cavalierly– contrived to contaminate his next ten suitable sarcoma cases with Streptococcus. His initial approach was to inject a solution of live bacteria deep into the tumour mass on a repeated basis over several months. The first patient to undergo this treatment was a bedridden man with inoperable sarcoma in the abdominal wall, bladder, and pelvis. Using this experimental method, the patient was cured spectacularly. He staged a full recovery, and survived another twenty-six years before dying from a heart attack. But subsequent results were mixed; sometimes it was difficult to get the infection to take hold, and in two cases the cancer responded well to treatment but the patients died from the Streptococcus infection.”
Well, it was only 1890 and as the paper claims in the fist link, medical science is pretty much complete shit on most cancer. They’d immediately apply antibiotics for a Strep, without even thinking of a possible upside.
…We’re over 300+ pages into the book, publisher loves. Hopefully we’ll talk soon.
Richard, immune-based therapy for cancer is what I did my post-doc in with Steve Rosenberg at NCI. You should definitely read his story: “The Transformed Cell” — one of the most important books I’ve read about science. The story of Coley is one of many, but unfortunately, to date, there seems to be a ways to go in this field. But when it works — which I’ve seen with my own eyes — it’s amazing. The immune system is beautiful.
Another great book re. Coley and the history of immune-based therapy for cancer is “Commotion in the Blood”
Indeed. I know Hall, also.
Hi Peter, please keep up the good work. What you’re doing with NuSi is vitally important.
I wish we’d known more about diet when my wife was diagnosed with GBM at the age of 34 (one week after the birth of our 2nd child) . She died 16 months later. We did ask about diet after her diagnosis, but the doctors thought it irrelevant. Note, she’d been a very high consumer of sugar all her life….
For the individual who referred to CMV infection and GBM – I actually met the lead researcher (done at Karolinska in Sweden) at a dinner party. There are a couple of things to remember, firstly, the extension of life with GBM is not necessarily a good thing – my wife asked to have her life ended four times. Secondly, my understanding was that they were looking at CMV as being causative in GBM – one of the samples they tested was not infected with CMV. My limited understanding would be that this would disprove the theory, unless you go for multiple mechanisms. Perhaps CMV could increase the likelihood/speed of progression….
We have to find a way of reducing the number of people who ‘get’ cancer rather than overly focusing on those with existing disease. I hope, ultimately, NuSi can help in the former.
This is so tragic. Can’t even imagine what your family went through. So sorry to hear this. Agree with your point about extending survival if quality of life is not improved. And I agree about prevention being the key.
Hi Peter,
Thank you for your insightful commentaries.
As a current patient with Hodgkins (nlphl), does this particular strategy (keto) add weight to treatment for immune system cancers? (I am athletic eat low carb and am in otherwise good shape).
Are there any brief suggestions you might add here to enhance treatment (abvd)?
I appreciate that you may be restricted from providing specifics and hope that I have not overstepped.
Thank you.
tw
This is not related to the essay on cancer metabolism. I’m very curious about the attached link, a story of a high fat (non-carb? certainly non-sugar) diet setting up/triggering a pre-diabetic condition. Weird.
https://edition.cnn.com/video/data/2.0/video/bestoftv/2014/02/04/newday-van-tulleken-twins-diet-experiment.cnn.html
The flaws in that show have been critiqued in this article and comments.
https://www.dietdoctor.com/sugar-vs-fat-on-bbc-which-is-worse
The Van Tulleken twins have some history on being oblivious to low carb.
Great article and makes the metabolism of cancer understandable for non-professionals (like me). I just finished the book “Cancer as a Metabolic Disease” by Dr. Thomas Seyfried and I must say it was a little too heavy reading. He has had some success especially with gliomas, but the glucose level in blood must be quite low: target 55-65mg/dl. To achieve that energy restriction is necessary (fasting). Glucose and Glutamine need to be decreased and fasting affects both. In addition to this ketosis is necessary. It reduces inflammation and replaces glucose with Ketone bodies, which can not be used by cancer cells as energy. The brain thrives using them. Ketosis needs to be at level 4 to 4.5 mM. Both targets are tough and it is obvious, that this therapy can not be applied to patients in a very weak condition.
From a practical standpoint, outside of a calorie-restricted KD, the way to get to sustained level of BHB at 4 mM likely requires new foods that also provided exogenous BHB and/or AcAc.
Re. Peter’s suggestion for foods that provide exogenous ketone bodies:
Richard Nikoley’s blog reports that potato starch, which is virtually all resistant starch, does not kick one out of ketosis and has no effect on blood sugar. It would make sense that not only is resistant starch not not anti-keto, but is perhaps pro-keto because, as I understand it, resistant starch fully ferments to short chain fatty acids in the colon, and is not at all absorbed in the small intestine.
Paul Jaminet’s blog proposes that specific amino acid isolates can supplement a keto diet to improve keto status and prevent muscle wasting. He reports that leucine and lysine are purely ketogentic and not at all glycogenic. Jaminet and various people in the muscle training world suggest that BCCA spare muscle on keto diets.
Maybe a good medium-term cancer keto strategy is a rather severe diet of little more than fat, MTC oil, BCAA (i.e. leucine plus the other required BCAAs), lysine, and potato starch? My thought is that this would drive extremely deep ketosis, maybe prevent muscle wasting while also providing protein restriction, and provide bulking and immune regulation to the colon.
> … the way to get to sustained level of BHB at 4 mM likely
> requires new foods that also provided exogenous BHB and/or AcAc.
You proposed this in at least one of your videos, as well, and in that case, I don’t think it was as a therapeutic adjunct. The proposal is pretty jaw-dropping, and I’m surprised it hasn’t generated more comment here. You’re proposing an entirely new type of human food (not just “supplement”, I note).
Speaking as a layperson merely interested in optimal diet, this raises a tsunami of questions, precisely none of which I expect you to spend any time answering in the near term.
Are we speaking of keto foods (KF) containing actual acetoacetate (AcAc) and ?-hydroxybutyrate (BHB), or just precursor compounds, as Jeff suggests above?
Can BHB and AcAc be directly ingested and passed into the bloodstream, or do they have to be protected from the upper GI?
What would KF replace in the dietary profile? Some fats, perhaps?
Would the product have a decent room temperature shelf life?
Any guesses as to any risks from over-consumption?
Would there be problems consuming KF on an otherwise non-keto, or even elevated glycemic diet?
I’m guessing that if ketogenic diet turns out to have a role in cancer treatment, that the most effective avenue to bring KF to market might be as a therapeutic agent. Whether or not there are patent opportunities here could affect the fortunes of that effort.
Actually, it would be great if it turns out to be something people can brew at home, so early adopters won’t have to wait out the commercial, political and bureaucratic sieges. The chances of a novel KF being considered GRAS from the outset appear to be nil.
Bringing KF to market as a supplement, much less a “food”, would be challenging, as you indicated in the video. Long-term food safety testing would be needed, and the usual glycemic confounding would likely need to be avoided (even though the food industry never does this for the junk they sell today).
No good deed…
Not directly relevant, but the saddest funniest thing I’ve read on how research is done:
https://www.edge.org/response-detail/25497
Edge asked 100 scientists what idea of science is ready for retirement, and Dean Ornish suggested the randomized controlled trial as a high standard (not that he doesn’t make some valid points about how they are done). Between the lines of what he says I hear what he doesn’t say, “RCTs are history because the outcome of one didn’t agree with my hypothesis that vegetable diet is it.”
Hi Dr. Attia,
The dysfunction of the mitochondrial complexes appears to ‘pollute’ the inner-membrane space with excessive ROSs & ‘electron leakage’…Do you suppose cancer could plausibly be an adaptation to such fuelling issues? (as often seen with T2DM patients for example) Or do you think the cause is more upstream?
Thanks!
PS: trying to square this with Peter @ Hyperlipid’s Proton Series…
Not sure, and it probably varies by cancer. There are likely many triggers to the problem.
If you’re looking for old discussions of the alternative hypothesis, I can beat that NYT article. Recently I was reading Herodotus’ Histories (circa 440 B.C.), and I had to laugh when I came across this passage:
“Finally [the Ethiopian king] came to the wine, and, having learnt the process of its manufacture, drank some and found it delicious; then, for a last question, he asked what the Persian king ate and what was the greatest age that Persians could attain. Getting in reply an account to the nature and cultivation of wheat, and hearing that the Persian king ate bread, and that people in Persia did not commonly live beyond eighty, he said he was not surprised that anyone who ate dung should die so soon, adding that Persians would doubtless die younger still, if they did not keep themselves going with that drink – and here he pointed to the wine, the one thing in which he admitted the superiority of the Persians.
“The [Persians], in their turn, asked [the Ethiopian king] how long the Ethiopians lived and what they ate, and were told that most of them lived to be a hundred and twenty, and some even more, and that they ate boiled meat and drank milk.”
Maybe it doesn’t have quite the rigor of a randomized controlled trial, but at least it’s interesting.
Classic finding.
Hi Peter,
are there any known results from clinical trials regarding the influence of a LCHF diet on the chances of getting cancer?
Great article, as always. “The Alternative Hypothesis” made me smile.
Not that I’m aware of. Primary prevention trials (what you describe) are almost unheard of in any disease, especially cancer.
Thanks for this article, Peter, I’ve been waiting. Because of a nasty cancer that was diagnosed at 39, which has since metastasized, I eventually went paleo and have recently started following Seyfried’s recommendations with good success. Your site has been extremely helpful to me in the past few years, and I look forward to digging into some of these links. Any and all articles you do about cancer are really appreciated.
I’m sorry to hear that Allison. One thing I would also consider is having your tumor specimen analyzed for insulin receptors. A few folks (Lew Cantly and Dom D’Agostino) are able to do this, though there is no commercial (i.e., CLIA) test for this. It would be a helpful step to be sure that your tumor is indeed sensitive to insulin (and therefore give you greater likelihood that this approach will be successful).
Another masterpiece Peter.
Simply, thank you again.
You take subjects I am fascinated by but do not have enough time to study (otherwise I would become a science junkie) and extract a concentrated form of meaning.
Information per word = density of Relativistic Heavy Ion Collider.
Thank you, JJ. I wish I could always write short pieces…most of the time I don’t have the time (cf. Mark Twain).
Thanks for your insightful post, Dr. Attia.
Tumor initiating cells appear to have enhanced glucose uptake mechanisms in brain cancer:
https://www.nature.com/neuro/journal/v16/n10/full/nn.3510.html
Could nutritional ketosis enrich for tumor initiating cells?
Tumor heterogeneity strikes me as a formidable challenge for therapeutic design and intervention.
Do you mean select for them? If so, why?
Summarizing from Flavahan et al., 2013 (Nature Neuroscience): Similar to hypoxia and acidic stress, glucose restriction appears to promote a brain tumor initiating cell phenotype in primary human cultures. Moreover, non-tumor initiating cells adopt a tumor initiating cell phenotype from this deprivation. The authors identified Glut3, a neuronal glucose transporter, as a mechanistic target of this enrichment, which has five times more affinity for glucose compared to Glut1. Glut3 is highly expressed in high grade gliomas and correlates with negatively with survival.
In the context of a calorie-restricted ketogenic diet, Glut3 mRNA expression decreases in rat neurons (Cheng et al., 2003, Endocrinology). Thus, tumor initiating cells may have a competitive advantage for glucose uptake in the brain.
With modest decreases in serum glucose during nutritional ketosis, brain tumor initiating cells may be epigenetically primed to respond to decreased glucose availability via Glut3 upregulation. Depending on the differential upregulation of Glut3, the net effect may be an increase in brain tumor initiating cells and paradoxical decrease in tumor volume since non-tumor initiating cells don’t express as much Glut3. This would potentially create a more aggressive tumor if a patient were to increase carbohydrate intake.
As a side note, I just moved to SD for a postdoc at TSRI. I’m also an avid cyclist and would love to meet up for a ride to discuss volunteer opportunities at NuSI!
Great review on the impact of diet on stem cell physiology: https://www.cell.com/cell-stem-cell/abstract/S1934-5909(14)00059-9
Caloric restriction appears to enhance self-renewal of stem cells, and presumably cancer stem cells.
Effects of ketogenic diets are unclear, but may be similar due to reduced insulin and IGF-1 signaling. With increased DNA repair, genomic stability, and immunosurveillance in this dietary state, the net effect on tumor incidence and growth would be very interesting to study.
What do you think the net effect would be?
As a nutritionist who’s beginning to get into helping people with low-carb and ketogenic diets for all sorts of conditions, I’d like to inject some general insights.
As Peter as suggested (with BHB of ~4mM and blood glucose in the 55-70 range), the ketogenic diet as adjunct therapy for cancer is a pretty different animal from a garden-variety low-carb diet. It is much stricter, and there are “extras” to throw in that can bump up ketones and lower BG a little (fasting, low-level activity like slow walking, liberal use of coconut & MCT products, etc.).
While the overall purpose of a KD for cancer is to “starve” the cancer while simultaneously nourishing *healthy* tissue (via ketone bodies and fatty acids), the ketogenic diet is most definitely not a miracle cure. I specifically used the phrase “adjunct therapy” because one of the good things about using a KD in conjunction with standard chemo/radiation treatments is that it seems to reduce the severity of the negative side effects of these. (Less GI distress, less nausea, less exhaustion — all of which might contribute to a patient’s appetite remaining strong enough to offset at least some of the cachexia.)
I’m not sure keto diets can do much *by themselves.* But they seem to add a powerful wallop to the conventional methods. What they’ve done in many cases is not eradicate the cancer, but extend life, and perhaps more importantly, improve the QUALITY of that extended life. A nutritionist named Miriam Kalamian has been in practice for years helping people implement keto diets for cancer. (She first got into it to help her son, who had a form of brain cancer. He eventually did pass away, but he was able to spend many more years with his family–*good* years–than anyone had predicted.) She has a lot of experience. You can check out her work here: https://dietarytherapies.com/
As for a KD being prophylactic, like another commenter said, time might tell. It would be interesting to get a prospective cohort of people together, keep them on a very low-carb diet for several years and see what happens. Pretty sure that study will never get funded, though. And as much as I love to hang my hat on low-carb, keto, Paleo, and all that, cancer is a wild, wild beast. It seems to “adapt” to, bypass, or override everything that gets thrown at it. A ketogenic diet is certainly a fantastic, science-based, logical, *non-toxic* intervention, but it’s likely not the magic bullet we want it to be. Some cancers seem to respond better to it than others.
That being said, we need a lot more research here, because even if it’s not a silver bullet by itself, clearly there’s *something* to the glucose/insulin/IGF-1 stuff, and I would love to see more research done on conventional treatments and pharmaceutical interventions in the context of a ketogenic diet, because the results might reveal very different (and presumably more promising) outcomes than when patients are following a Standard American (read high-carb, high seed oil) diet, or worse — chugging Ensure shakes, ice cream, or whatever they can keep down per their conventional oncologists just to “keep the calories up.”
Great points, Amy. I, too, believe that KD in its strictest form (calorie restricted with exogenous ketones keeping K/G > 1), plus other modalities such as hyperbaric O2 and select chemo might be the ultimate strategy, but this my speculative opinion based on biologic intuition and animal data.
> … exogenous ketones …
Looks like EKs all by themselves have a material effect:
“Ketone supplementation decreases tumor cell viability and prolongs survival of mice with metastatic cancer.”
https://www.ncbi.nlm.nih.gov/pubmed/24615175
Summarized by Patrick Arnold at:
https://patrickarnoldblog.com/can-ketone-supplementation-cure-cancer/
I’m guessing that Patrick had some role in formulating the EKs used.
Dear Amy, this is Carmen from Peru. I am investigating about KD cause I am a medical doctor and an oncology patient I don’t know if it could be possible that you bring me some way for contact with Miriam Kalamian. Thank you very much and excuse my spanglish.
Carmen
Amy,
I agree that KD is at best adjuvant therapy against cancer. There’s a 2015 paper on Chen.Sci. that seeks to exploit the large difference in Delta Psi (the mitochondrial membrane potential) between regular cell in KD (low voltage) and cancerous, glycolytic cells (high voltage). I don’t have the details but the researchers plan to deliver 3-bromopyruvate, the so-called Trojan Horse of energy substrate, to the latter to destroy them.
How effective is 3-BMP versus Peter Pedersen’s John Hopkins group’s claims is a side note to my point that the large difference between Delta Psi is a unique property and should be fully exploited.
John
“The energy blocker inside the power house: mitochondria targeted delivery of 3-bromopyruvate”
Steve,
I just stumbled onto this blog. Love it. I’m a PhD scientist studying cardiovascular health and only recently (embarassingly) really come to understand diet, exercise, and the role of carbs et al in their ‘proper light.’
This and other posts are fascinating. I recently had the pleasure of hearing Lew Cantley talk about the use of PI3K pathway inhibitors (many companies are pursuing these) to shut down the Insulin/Glut4 mechanism and starve cancer cells (I’m guessing you’re aware of this story though you don’t mention it here). Flip side of the coin from attacking cancer by diet (see question from Anonymous above). I’m not at all sure diet/glucose starvation/PI3K inhibition of cancer is headed for a cure (maybe for some), but I can imagine it having a dramatic effect on at least slowing progression (for many).
Thanks for a great blog. Some of the most valuable time I’ve spent on surfing the net in a long while.
Jimmy Moore has an interview he is re-running with Dr. Thomas Seyfried on his LLVLC Show today, with the topic of ketogenic diets for cancer (episode 777).
Just recently research has proven that cancer is not a disease of the DNA. I wonder how long it will take until these results are accepted. This article is particularly interesting;
https://robbwolf.com/2013/09/19/origin-cancer/?utm_content=buffer80ddb&utm_source=buffer&utm_medium=twitter&utm_campaign=Buffer
Having read Explanation 1 and explanation 2, is there anything in our diet that damages mitochondria or causes genetic insult?
Probably.
> … is there anything in our diet that damages
> mitochondria or causes genetic insult?
The context for this question, I presume, is that although the later stages of most cancers may have a common etiology (fermentation metabolism), the triggers for the initial cellular upset are myriad.
I expect that the list of triggers would include things we might ingest. Obviously this includes food contaminants known to be carcinogenic such as dioxins, benzene, etc. You already avoid those. Any number of food additives are popularly suspect (and a diminished issue if you eat raw foods, although I consider pesticide/herbicide uptake and GMO to be open questions). I further assume triggers include inflammatory foods of many sorts (e.g. Omega 6 industrial seed oils), as well as foods that cause intestinal permeability and are apt to be the provokers of auto-immune problems, such as gluten-bearing grains, and the zein protein of corn. I consider nitrites to be an open question. Acrylamide is avoided by avoiding high temperature cooking. This is far from a complete list.
Does excess sugar itself pose a trigger threat? Great question, and it might be multiple threats (as it adds insulin resistance which may set cells up to become cancerous, then provides the actual cancer fuel).
Interestingly, an LCHF diet (with attention to which fats, as well as focus on substantial raw food content) is apt to both be very low in trigger foods, and provide some benefit from the low and stable glucose level in the blood. We may not know for some time just how protective LCHF is against cancer, so remain vigilant about triggers.
Mainstream cancer research, of course, is still largely stuck in the somatic (gene) theory, and there’s a hazard there. If they can’t figure out how a potential adverse agent might cause DNA damage, they tend to deprecate its carcinogenic potential.
With cell phones, for example, the claim is that the radiation is too low to be ionizing. But what if the real hazard is merely thermal – standing waves overheating mitochondria for example. Anyone concerned about this can substantially mitigate the thermal hazard by using a BlueTooth headset (which emits 1/100th the RF power vs. cellphone) and by not keeping the actual phone against your body all day long. A tinfoil hat probably doesn’t provide much protection. 🙂
“…is there anything in our diet that damages mitochondria or causes genetic insult?”
Norm,
Peter at Hyperlipid writes a lot about this topic and has many in depth articles if you are so inclined. Here is a link to one of many articles in the proton series about how hyperglycemia causes mitochondrial damage:
https://high-fat-nutrition.blogspot.com/search/label/Protons%20%2826%29%20Chowdhury%20and%20Crabtree%20play%20with%20mitochondria