Before I get into this post I want to lay a few things out.
- This post is written mostly for doctors, but also for patients who really want to understand this topic, if for no other reason than to help them choose the right doctors. I don’t go out of my way to simplify the terminology and I assume the reader is familiar with the topics covered in the cholesterol series I wrote. If you encounter a term you don’t understand, Google is a pretty good place to find the definition.
- I will not use this post to in any way get into prescriptive strategies, which involve modifications of nutrition, hormones, and yes, lots of drugs across four or five classes (i.e., much more than just statins) depending on the specific situation at hand and the risk appetite of the patient and physician, as well as the other comorbidities that must be co-managed. Even if I wanted to write out all of my prescriptive leanings I could not do it briefly.
- Please do not email your lab results or ask me to weigh in on your case. You know the disclaimer: I can’t practice medicine on a blog or over email.
There was a day when the only thing I argued about was who the greatest boxer of all time was. (I’m fighting all urges to turn this post into a manifesto of 1965-67 Muhammad Ali vs. 1938-41 Joe Louis vs. 1940’s Sugar Ray Robinson vs. 1937-42 Henry Armstrong.)
Today, however, I find myself arguing about so many things—some of them actually important—from why symptomatic women should receive hormone replacement therapy after menopause (and, by extension, why the Women’s Health Initiative tells us so, if you know how to read it) to why monotherapy with T4 for hypothyroidism is a recipe for disaster for most patients. But there is nothing I find myself debating more than the misconceptions most doctors have about heart disease. This is especially troubling since heart disease kills more Americans than any other disease. To put this in perspective, a woman in the United States is 7 to 8 times more likely to die from heart disease than she is from breast cancer.
Here are the typical arguments put forth, almost always by doctors, which invariably result in my need/desire to counter:
- Heart disease is caused by too much “bad” cholesterol (LDL-C).
- LDL-C is the only target of therapy you need to worry about.
- Calcium scores and CT angiograms (CTA) are great ways to further risk-stratify (the corollary: when these tests are negative, there is no need to treat the patient).
- Atherosclerosis is a “pipe narrowing” disease (ok, nobody uses these words, but they imply this by saying it’s a luminal narrowing disease).
- There is no role for preventatively treating young people, except in very rare cases like familial hypercholesterolemia.
Briefly, here are my counters:
- Atherosclerosis is caused by an inflammatory response to sterols in artery walls. Sterol delivery is lipoprotein-mediated, and therefore much better predicted by the number of lipoprotein particles (LDL-P) than by the cholesterol they carry (LDL-C) [Bonus point: always measure Lp(a)-P in your patients—but we’ll have to save Lp(a) for another day; it certainly owns its own blog post].
- Ditto point #1. And don’t ever bring up LDL-C again.
- Calcium scores and CT angiograms of exceptional quality (the operative word being exceptional—most are not) are helpful in a few settings, but this assertion is patently false, and I will leave this discussion for another blog post as the topic is too rich in nuance for a few lines.
- We’ll discuss this today.
- By necessity, we’ll be forced to confront this today, also.
Before diving into this topic it’s really important for me to acknowledge the person who has taught me almost everything I know about this disease, beginning back in 2011 when I first became aware that I basically had no idea what atherosclerosis was. For many years Dr. Tom Dayspring’s generosity has been remarkable and I’m humbled to be his most sponge-like student. Tom has not only given me an on-the-side lipidology fellowship, but he has also introduced me to the finest lipidologists and cardiologists in the country who have, in turn, been incredibly generous with their time and knowledge. I’m not the only one to benefit from Tom’s wisdom and generosity. I had dinner with Tom’s son and his wife once and I described Tom to them as a national treasure. That’s really how I feel about him. He is a nationally-recognized educator and his writing and presentations are devoured by fanatics like me across the globe. With Tom’s permission, I’ve deconstructed a video he put together into a series of figures which I’ll use to begin this discussion of how atherosclerosis actually takes place.
The physics of luminal narrowing
Traditionally, the atherosclerotic process was believed to involve plaque accumulation that prompted the gradual narrowing of the lumen, with the eventual development of stenosis. Stenosis then caused impaired control of flow (stable angina) and plaque rupture and thrombosis (unstable angina and MI). Consequently, prevailing opinion held that coronary angiography would be able to gauge the atherosclerotic process at all stages of disease.
However, in 1987, Glagov and colleagues proposed an alternative model of atherosclerosis development. After performing histological analyses of coronary artery sections, Glagov et al. reported that early atherosclerosis was characterized by plaque accumulation in the vessel wall and enlargement of the external elastic membrane (EEM) without a change in lumen size.
As atherosclerosis progressed, they found that plaque continued to accumulate in the vessel wall until the lesion occupied approximately 40% of the area within the EEM. At this point, the lumen area began to narrow. These findings have since been confirmed by intravascular ultrasound (IVUS). Due to the complex remodeling that occurs in the earlier stages of atherosclerosis, coronary angiography, which only visualizes the lumen, tends to underestimate the degree of atherosclerosis. In other words, atherosclerosis is well under way long before angiography is able to identify it.
I was reminded of the words of my Pathology professor back in the first year of medical school, “The only doctors who actually understand atherosclerosis are pathologists.” I would add lipidologists to that list, but I saw his point.
Most people, doctors included, think atherosclerosis is a luminal-narrowing condition—a so-called “pipe narrowing” condition. It’s true that eventually the lumen of a diseased vessel does narrow, but this is sort of like saying the defining feature of a subprime collateralized debt obligation (CDO) is the inevitable default on its underlying assets. By the time that happens, eleven other pathologic things have already happened and you’ve missed the opportunity for the most impactful intervention to prevent the cascade of events from occurring at all.
To reiterate: atherosclerosis development begins with plaque accumulation in the vessel wall, which is accompanied by expansion of the outer vessel wall without a change in the size of the lumen. Only in advanced disease, and after significant plaque accumulation, does the lumen narrow.
Michael Rothberg wrote a fantastic article on the misconception of the “clogged pipe” model of atherosclerosis. He opens with the following story:
A recent advertisement on the back cover of a special health issue of the New York Times Magazine section read “Ironic that a plumber came to us to help him remove a clog.” The ad referred to doctors in the cardiac catheterization laboratory as “one kind of pipe specialist,” and noted that the patient in the ad returned to work “just 2 days after having his own pipes cleaned out.” Although the image of coronary arteries as kitchen pipes clogged with fat is simple, familiar, and evocative, it is also wrong [emphasis mine].
Dr. Rothberg goes on to explain that for patients with stable disease, local interventions can only relieve symptoms; they do not prevent future myocardial infarctions. To be clear, at least 12 randomized trials conducted between 1987 and 2007, involving more than 5,000 patients, have found no reduction in myocardial infarction attributable to angioplasty in any of its forms. And yet, despite this overwhelming evidence, the plumbing model, complete with blockages that can be fixed, continues to be used to explain stable coronary disease to patients, who understandably assume that angioplasty or stents will prevent heart attacks—which they patently do not.
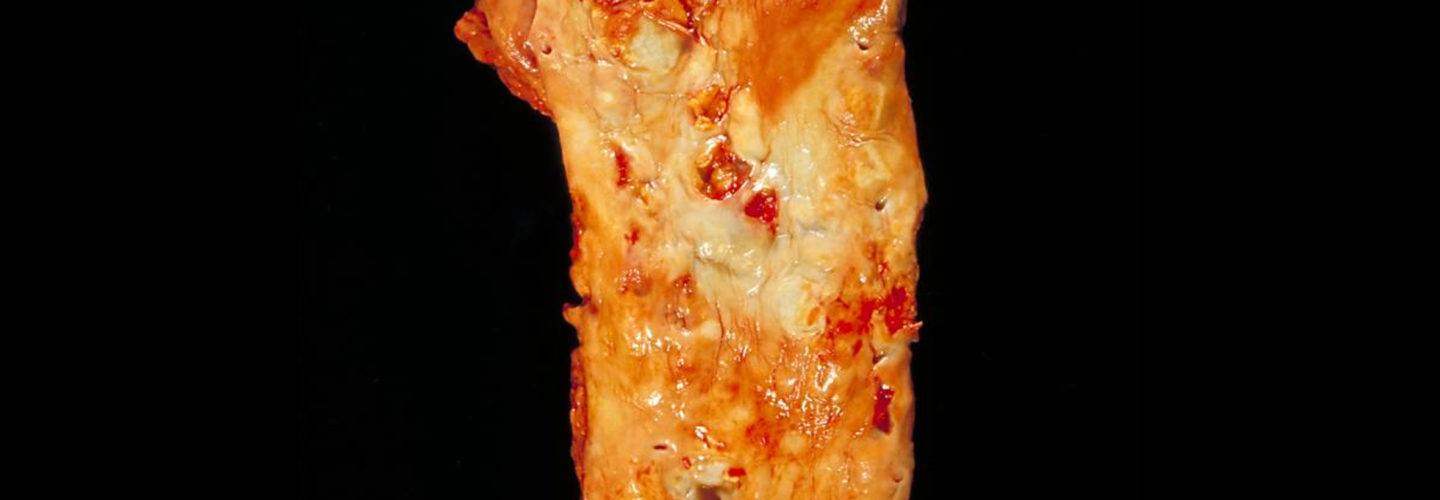
The root of the problem, in my view at least, is that we as doctors—and by extension, our patients and media—spend too much time looking at images like this (angiograms of coronary arteries complete with “clogged pipes”):
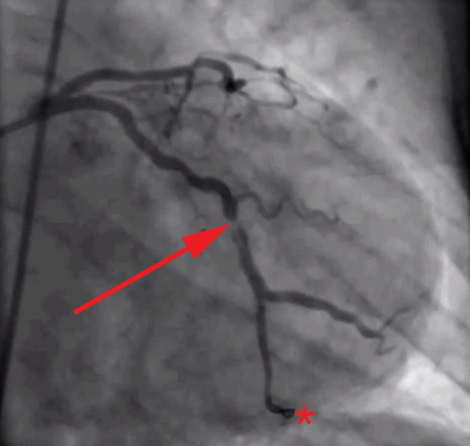
And not enough time looking at images like these (the histological, i.e., pathology, sections of coronary arteries):
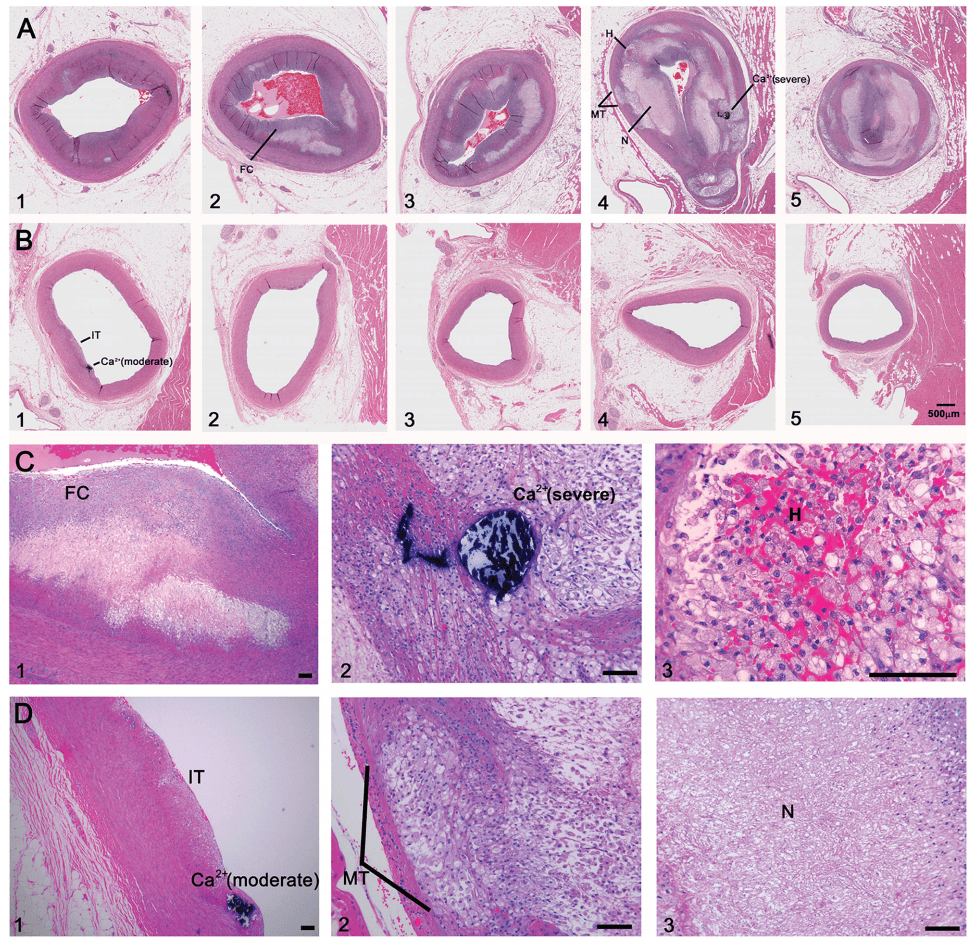
But who can blame us, I mean, angiograms are cool! But, alas, it’s time to get serious about understanding this disease if we want to prevent/delay it.
Atherosclerosis, for the cognoscenti
Ok, so now let’s get rigorous about the pathology that kills more Americans than any other disease. To understand this, as Frederic Bastiat wrote long ago, we must resort to “long and arid dissertations.” Buckle up.
The following figures were constructed from a video Tom Dayspring produced in one of his stellar lectures on the development of atherosclerosis. I’ve broken the video down into 20 or so steps which show the transition from a completely normal endothelium (i.e., at birth) through myocardial infarction. Each figure is preceded by a brief explanation of its content.
The endothelium is a protective one cell layer lining the surface of the artery lumen. Endothelial cells perform many complex functions and are capable of modulating vascular tone, as well as inflammatory and thrombotic processes. Their function depends on many circulating and local factors.
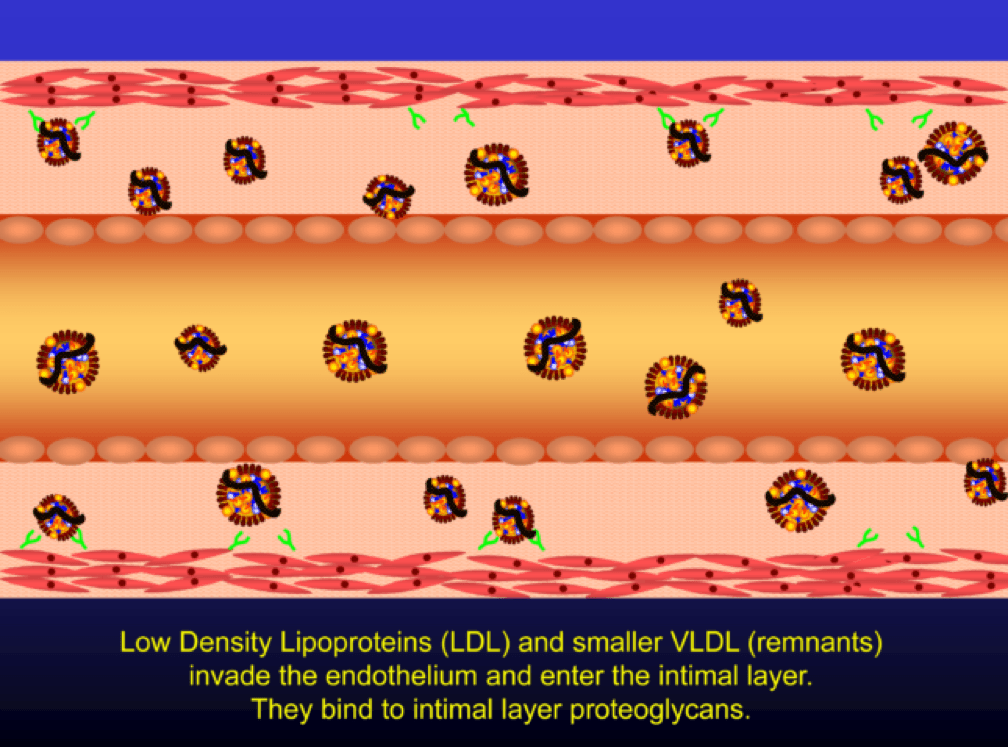
Low density lipoprotein (“LDL”) is a lipid (the bulk of which is cholesterol) transport particle. Please re-read this sentence. It is not “bad cholesterol,” a term that has no meaning. LDL—the particle—allows lipids (cholesterol, but also triglyceride, phospholipid) to be delivered through the aqueous medium of the blood, since lipids are hydrophobic (i.e., repel water) and a “carrier” is needed to transport them in blood (which is mostly water).
If LDL particles are present in physiologic (i.e., normal) concentrations, they effectively deliver cholesterol to those tissues that require it (recall: all tissues make cholesterol but some don’t make enough for their own needs and therefore cholesterol needs to be trafficked around the body).
The term LDL particle, LDL-P, and apoB are used interchangeably (the latter, because LDL particles are defined by the wrapping of a lipoprotein called apolipoprotein B-100).
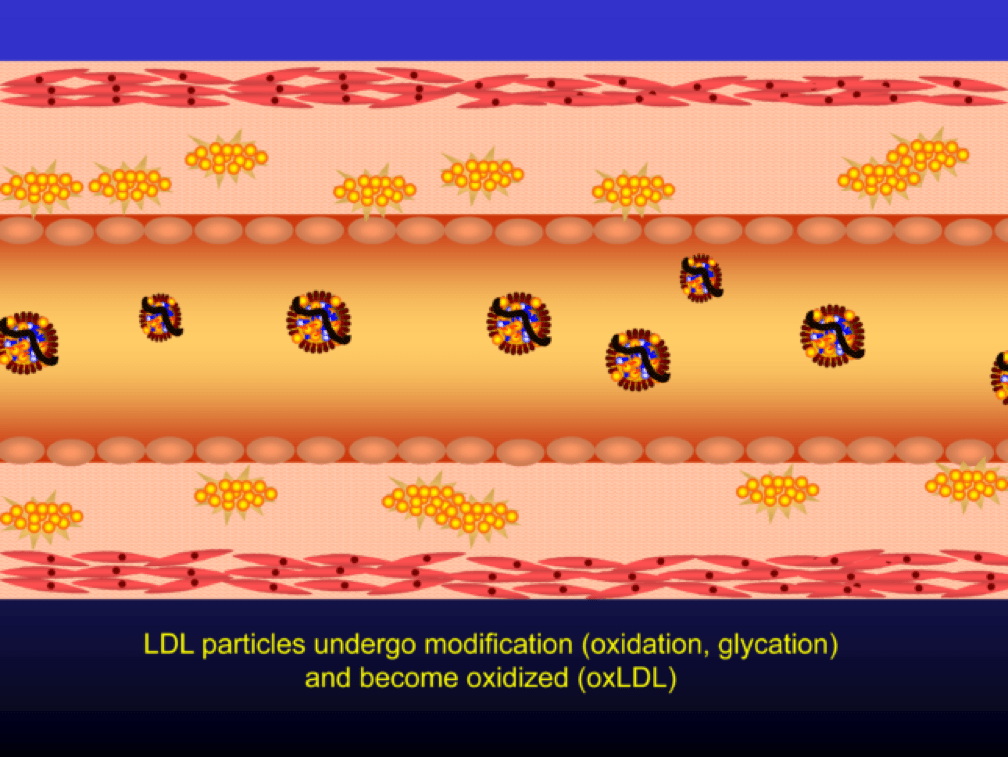
When LDL particle concentration is elevated, the lipoprotein penetrates into the subendothelial space. Once in the intimal layer, they are securely attached to intimal proteoglycan molecules. The first step in atherogenesis is surface phospholipid (PL) exposure to reactive oxygen species and oxidation of the PL. LDL particles that are not oxidized are not atherogenic. To be clear, it’s not the “getting in there” part that is the problem (HDL particles do this all the time and so do LDL particles, for that matter), it’s the “getting stuck and oxidized in there” part, formally known as retention and oxidation.
Once retained in the subendothelial space, the LDL particle may be modified, or oxidized (the clusters of yellow circles in the subendothelial space, below).
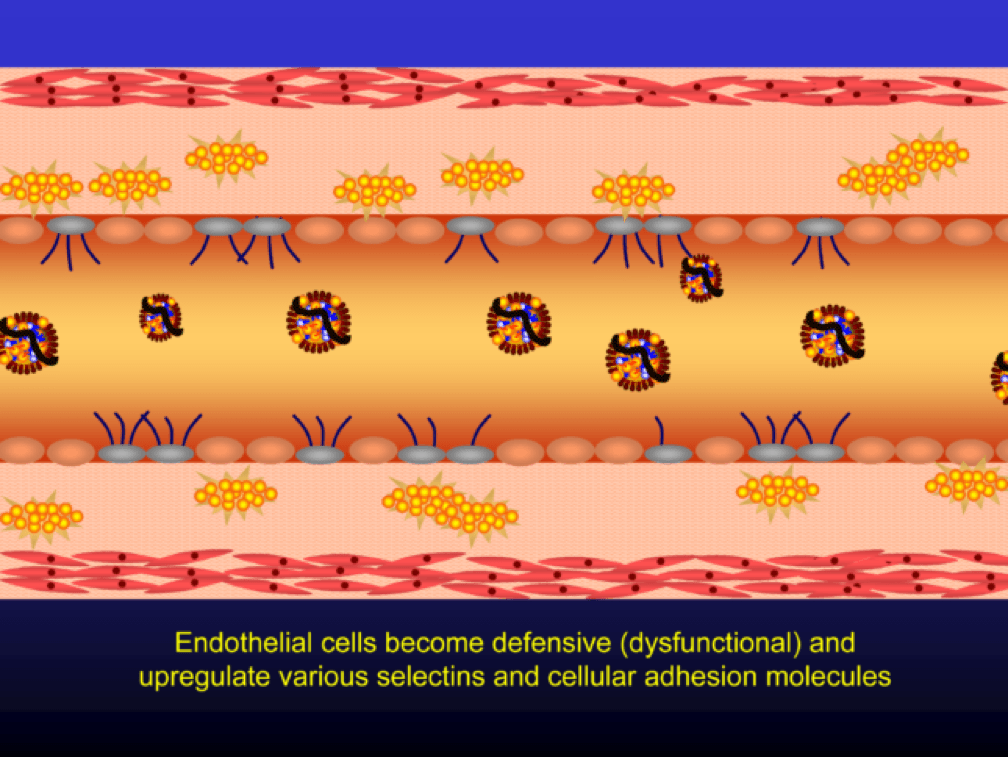
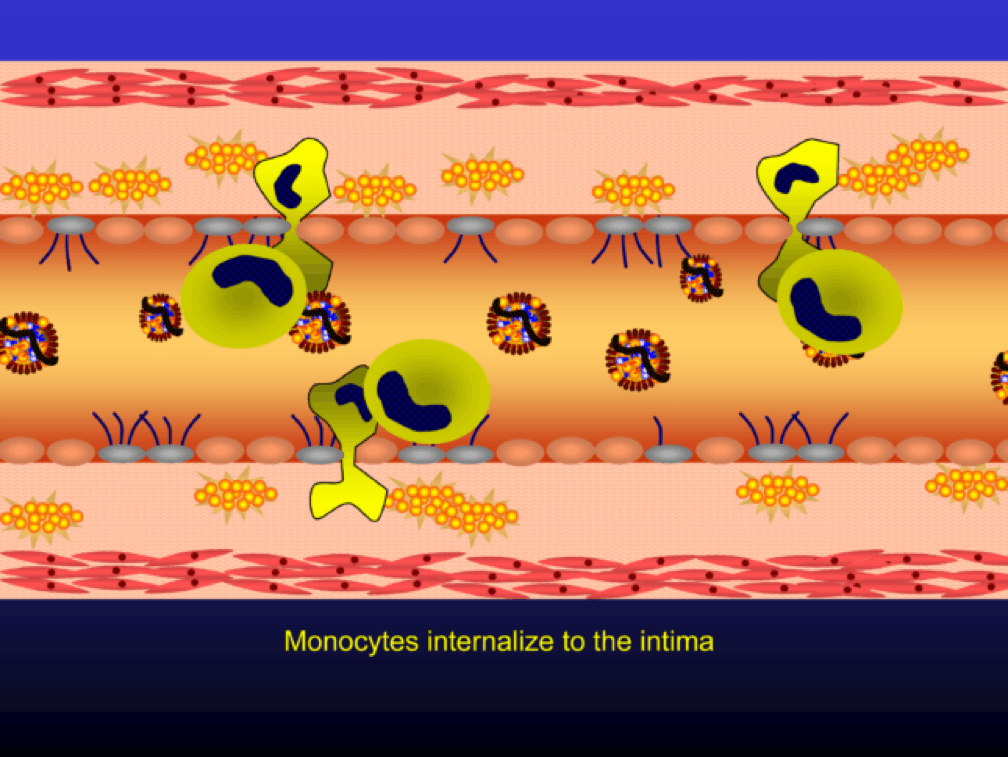
Oxidized LDL particles are toxic to the endothelium. Now-dysfunctional endothelial cells express selectins and vascular cell adhesion molecules (VCAMS) which mark the injured areas of the vascular wall. It is worth pausing here for a moment. This step is kind of the turning point in the story. It’s also a perfectly “normal” thing for the epithelium to do. They sense a problem and like any law-abiding tissue, they ask for help from law enforcement. Think of the selectins and VCAMS as 911-calls. The police, who show up shortly, are the monocytes.
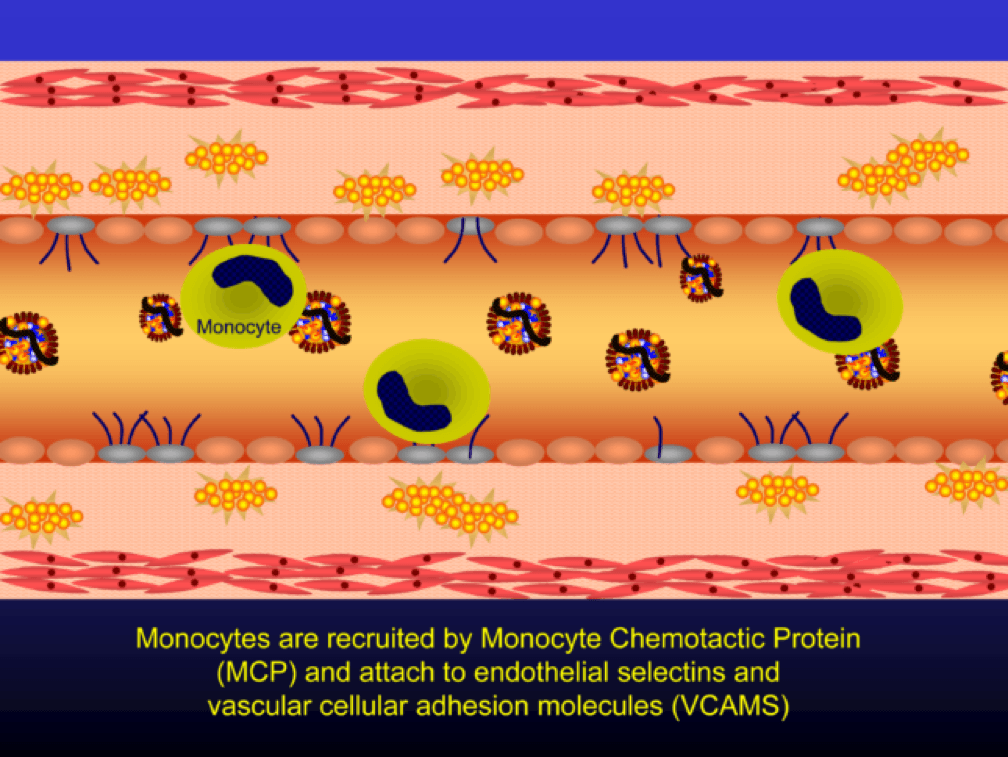
Selectins and VCAMS increase monocyte adherence to the endothelium.
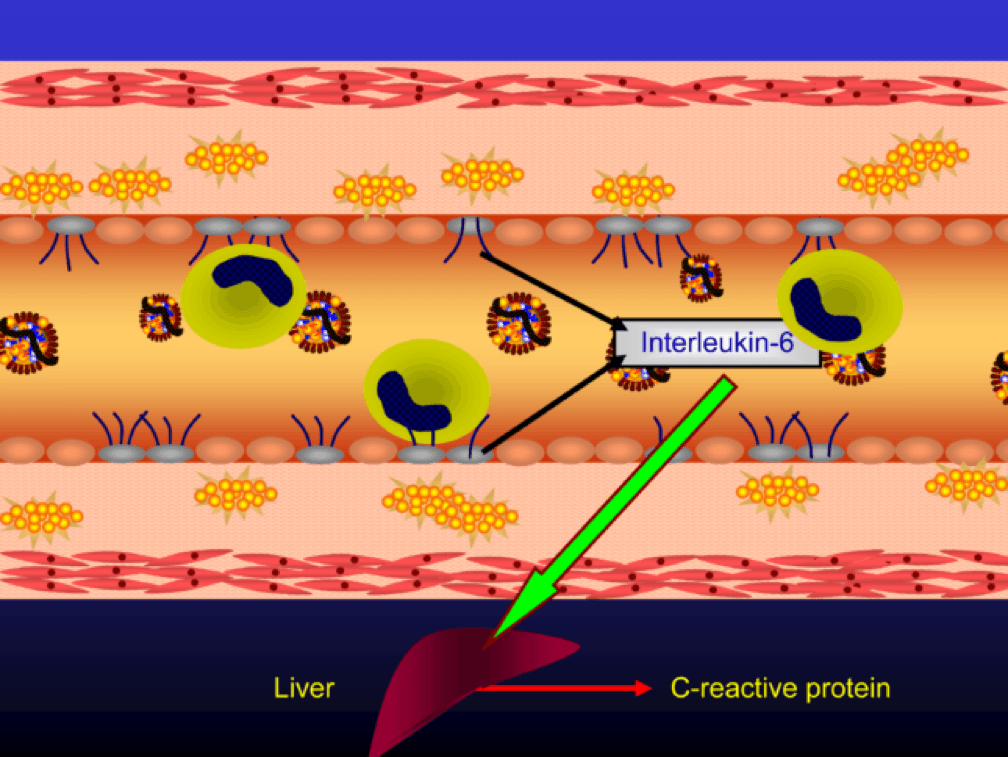
The endothelial cells also express messenger cytokines such as interlukin-6 (IL-6) and tumor necrosis factor (TNF) which circulate to the liver and induce the production of C-reactive protein (CRP).
Monocytes penetrate the subendothelial space…
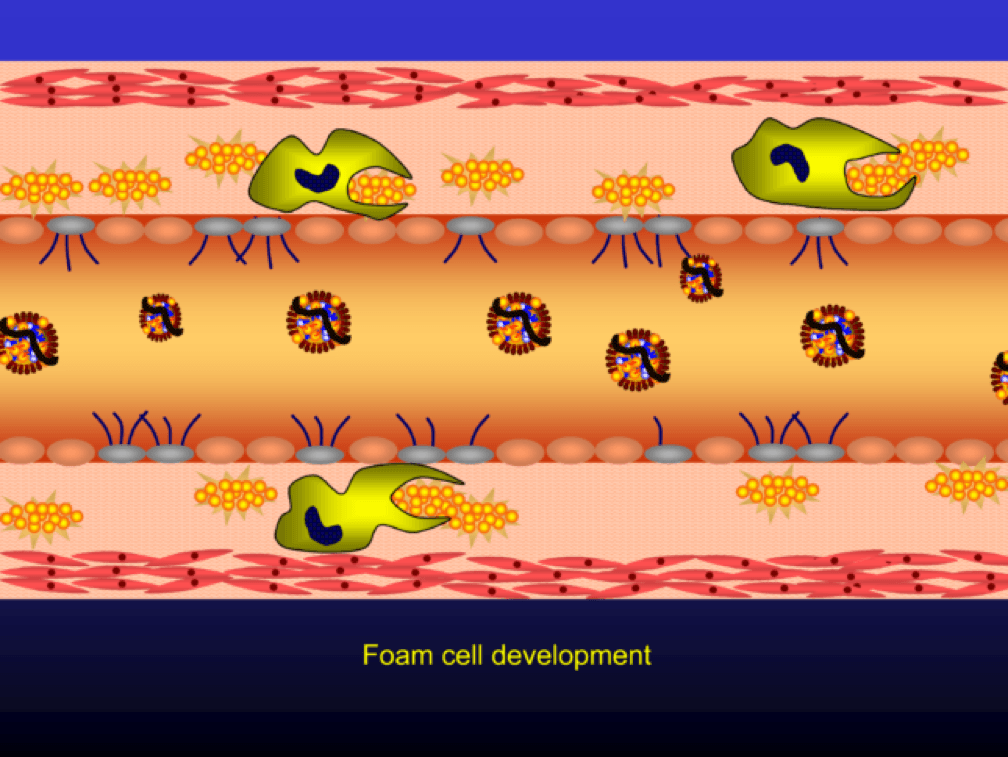
…and when they do, the monocytes differentiate into (i.e., “become”) macrophages (a more specific type of immune cell). Macrophages phagocytize (basically “ingest”) the modified or oxidized LDL particles.
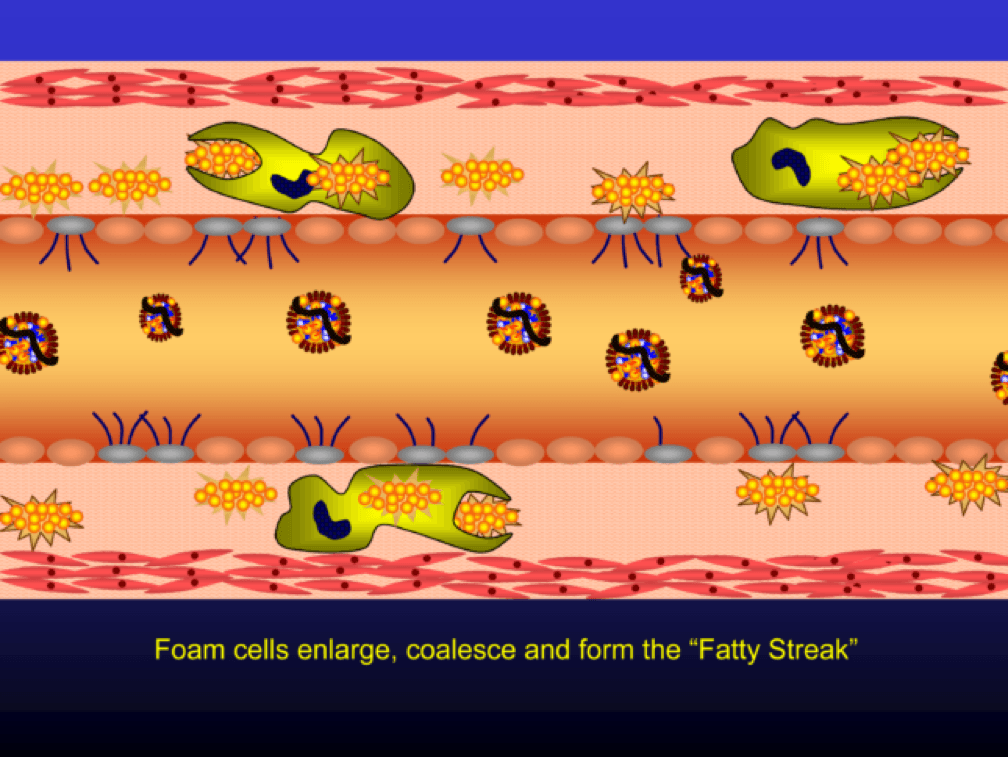
The phagocytosis of oxidized LDL particles (oxLDL) and accumulation of lipid in the macrophage creates something called a foam cell.
Multiple foam cells coalesce to form the characteristic fatty streak, the hallmark of an early atherosclerotic plaque. Keep this in mind. Later in this post we’ll come back to “fatty streaks” and I want you to remember how much has taken place to get us to this stage. I will posit that no one reading this post does not have fatty streaks unless there are some brilliant 5-year-olds reading this during recess..
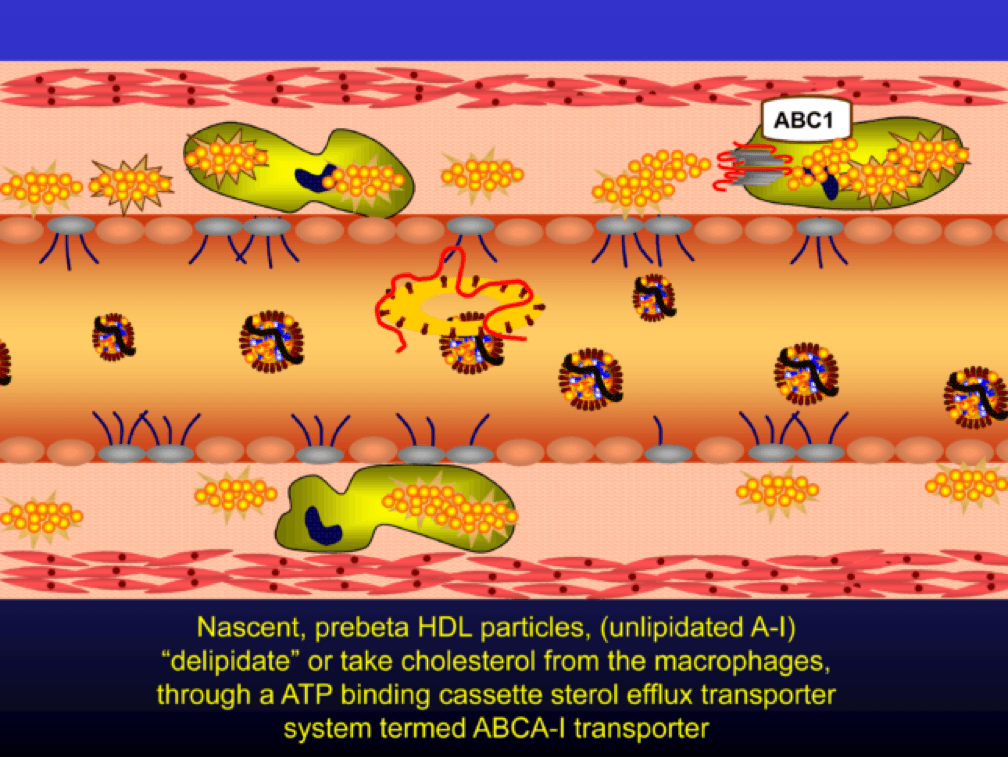
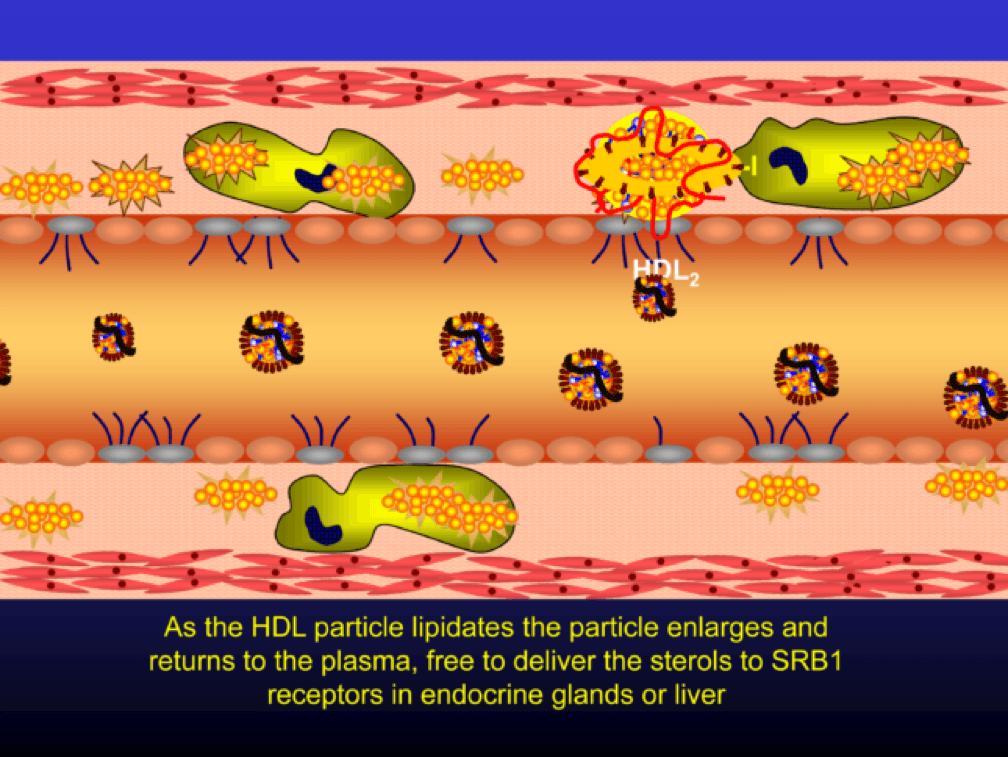
Nascent Apo A-I containing particles (also known as prebeta-HDL particles) accumulate free or unesterified cholesterol from the macrophages using ATP Binding Cassette Transporters A1.
As the HDL particle lipidates itself by delipidating the macrophage (i.e., takes lipid out of the foam cell), the HDL particle, utilizing an enzyme called LCAT, esterifies the free cholesterol forming cholesteryl ester (CE). As a result, the HDL particle enlarges and is free to be delipidated at a variety of tissues. HDL delipidation occurs through multiple mechanisms using Cholesteryl Ester Transfer Protein (CETP), aqueous free diffusion, SRB1 receptors in endocrine glands or gonads (e.g., to make hormones), adipocytes (a major cholesterol storage organ) or the liver (e.g., to package for biliary delivery). HDLs can also be internalized as entire particles by surface liver receptors.
A brief, but important, digression: the complexity, above, is probably the reason why every trial that has tried to increase the concentration of cholesterol in HDL particles (i.e., raise HDL-C) has failed, and failed epically, to reduce events. The value in HDL particles (the so-called “good cholesterol”—my god I hate that term as much as I hate the term “bad cholesterol” when referring to LDL) is almost assuredly in its functional capacity—what it is doing that might be cardioprotective (very hard to measure) rather than its cholesterol content (HDL-C), which is relatively easy to measure, but probably offers zero insight other than its positive epidemiological associations. In other words, measuring HDL cholesterol content tells you little about its cholesterol efflux capacity or any other of the numerous HDL functional properties.
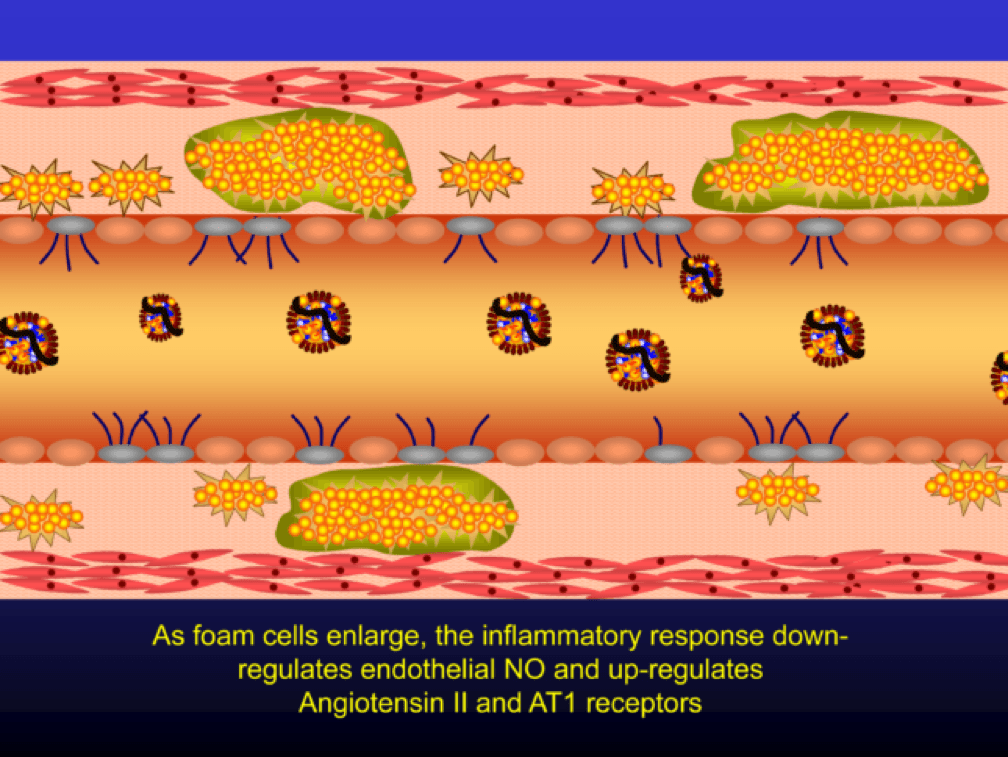
Macrophages that become engorged with oxLDL remain full-fledged foam cells. Foam cells produce Angiotensin II, metalloproteinases, collagenases, elastases, and other proteins, which undermine the integrity of the arterial wall, causing more endothelial dysfunction. This is where the process goes to hell. Now you’ve got a damaged barrier and the looting begins. Oh, by the way, the process to date goes unnoticed by the calcium scan and CTA.
Chemotactic factors (i.e., chemical signals) trigger migration of smooth muscle cells to the area of injury in an attempt to repair the damage and pervert further disruption. Again, all of this is taking place in good faith on the part of the immune system. The smooth muscle cells are transformed into secretory cells that lay down a matrix to heal the injured wall. This matrix becomes the fibrous cap of the atherosclerotic plaque. Only now is the arterial lumen becoming encroached.
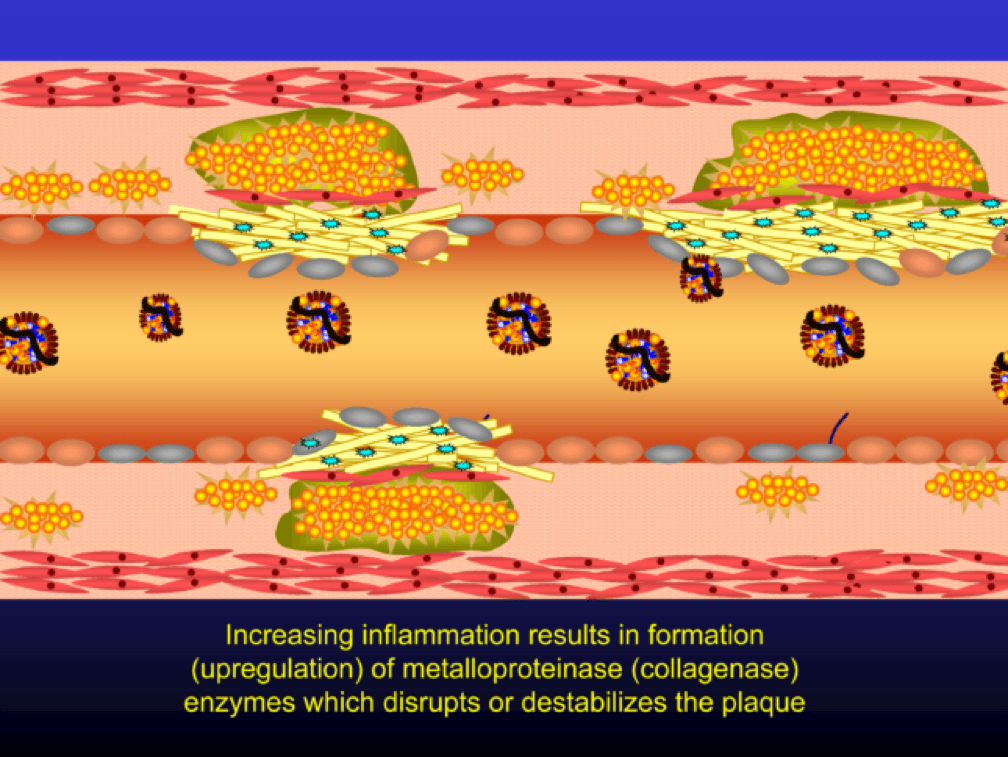
Metalloproteinases and collagenases are upregulated and they start to dissolve or weaken the plaque cap, typically at the shoulder regions where the diseased endothelial cells meet healthy endothelial cells. Some have used the term “vulnerable” to describe such plaques, which may be a correct term, but it also gives a false sense of confidence that we can treat atherosclerosis on a lesion-by-lesion basis. History has taught us that such hubris is unwarranted. Until proven otherwise, atherosclerosis should be viewed as a systemic condition of the arterial system. To see one of the best (and my favorite) papers on this topic, look no further than to this paper by Armin Arbab-Zedeh and the venerable Valentin Fuster, aptly titled “The Myth of the Vulnerable Plaque.”
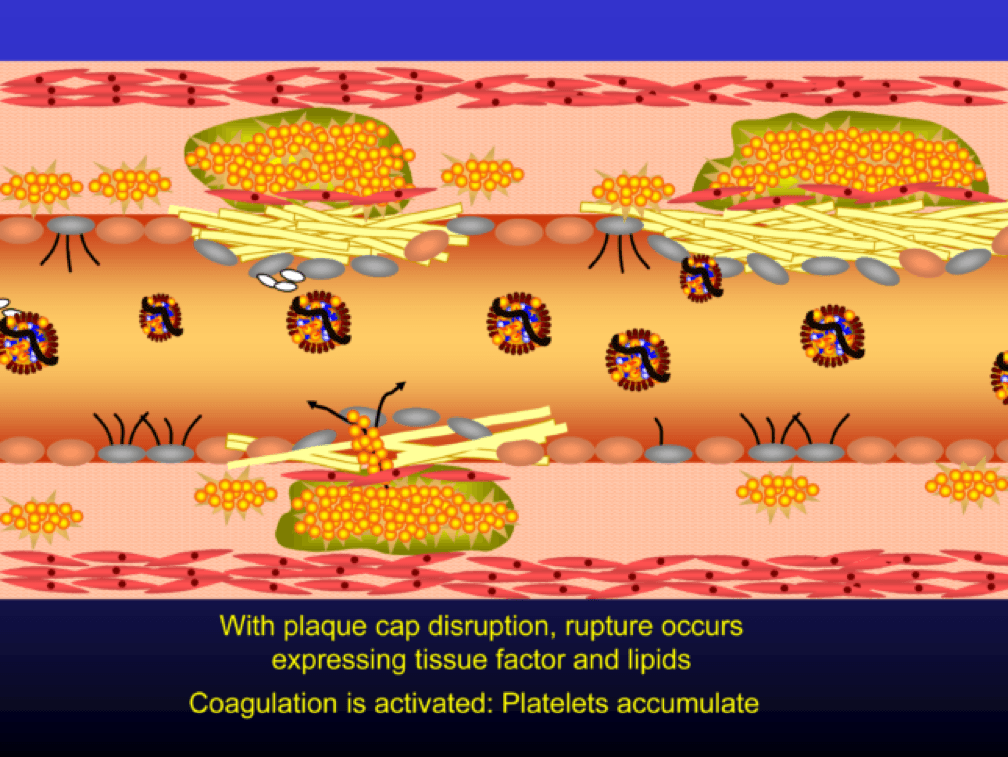
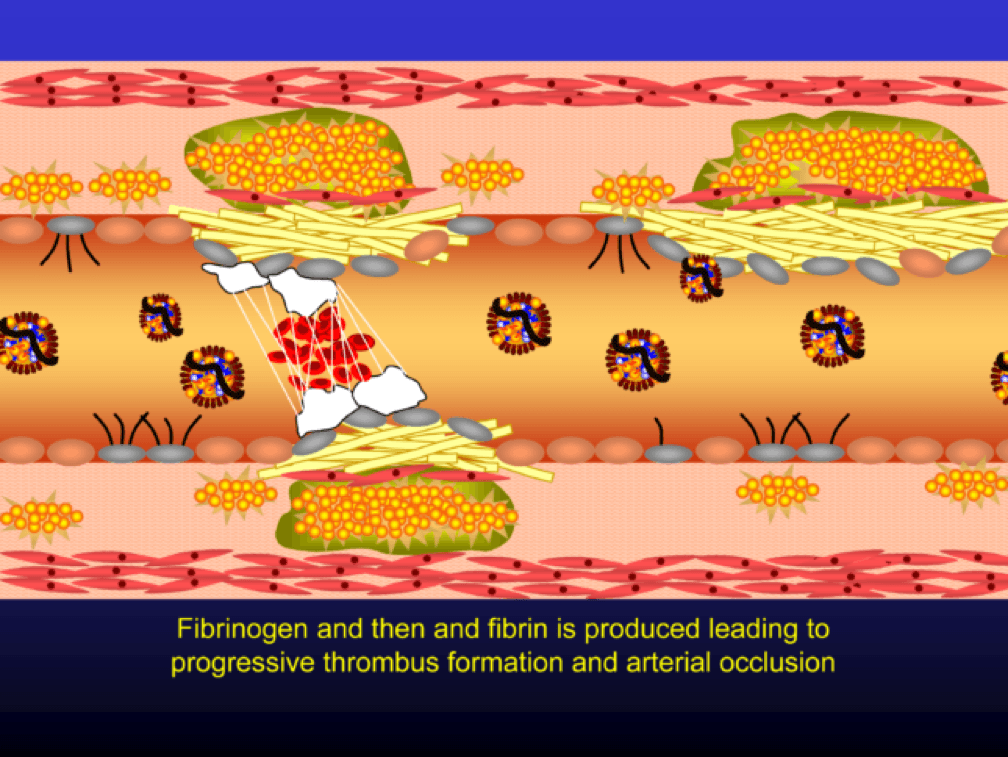
The plaque can become obstructive—i.e., it can obstruct the lumen—over time. Lipid rich plaques are unstable, and can rupture. Platelets adhere to the ruptured surface of the plaque through electrostatic factors and through binding to specific ligands.
The platelets then serve as a cradle for the coagulation cascade to produce a net of fibrin (the white “net” in the figure), leading to a red clot, as red blood cells are caught in the net. A non-obstructive plaque can lead to a clinical event after the superimposition of red and white thrombus, which can occur quickly and without warning. The degree of stenosis (luminal narrowing) does not predict when this will happen.
Why all of this matters
So back to this impetus for this post—Allan’s editorial. When I first met Dr. Allan Sniderman, we immediately hit it off. Like Tom, Allan has been a remarkable mentor and teacher. In fact, one of the gifts Allan gave me a while back was his personal copy of Herbert Stary’s legendary pathology textbook on atherosclerosis, Atlas of Atherosclerosis Progression and Regression. I devoured this textbook and have since purchased additional copies to always have one on hand. Ask my patients…most of them have had to sit through viewings of it like it was a vacation photo album.
Allan and I were having dinner and discussing our favorite topic. Allan asked me to guess what fraction of cardiac events (“event” is a pretty common word in the vernacular of cardiovascular disease and basically refers to a Q-wave MI, the need for re-vascularization, or cardiac death) take place in North America in those younger than 65. I knew it was a loaded question, so I rounded my guess up to 25%. I was wrong. How wrong I wouldn’t find out until Allan and his colleagues completed their analysis which formed the basis for their editorial recently published in JAMA Cardiology. Add it to your weekly reading list.
You don’t have to be a lipidologist or a pathologist to understand this paper, but it helps to understand the basics of math—big denominators can drown out even modest numerators. The aggregate figure from this paper is one of the most elegant representations of a dot product. The subfigure on the left is how most doctors (myself included until the 2nd decade of the 21st century) and authorities think of coronary heart disease (CHD)—it’s virtually silent until the 7th or 8th decade of life. What folks who think this way miss is the middle figure—the population base (the “denominator”) is shrinking while the incidence (the “numerator”) is rising. In a situation like this, the only way to really see what’s happening is to do what Sniderman et al. did—calculate the absolute event rate, as shown on the right.
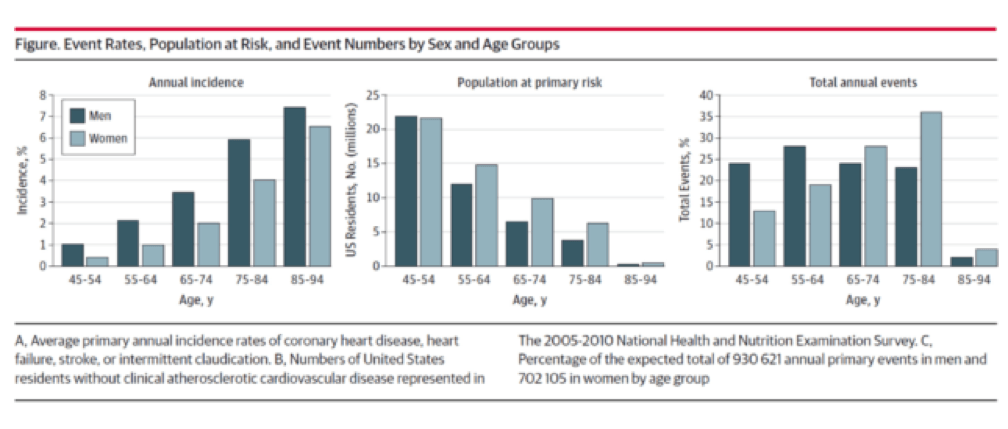
Yes, you’re reading this graph correctly. A little over half of all events in men (24% + 28%) and a little less than a third of events in women (13% + 19%) take place below the age of 65.
Tying these two insights together
Insight #1: Atherosclerosis takes a long time to evolve, and involves many steps.
Insight #2: Many cardiovascular events—half in men and one-third in women—take place in young people (i.e., those 64 or younger)
How do we reconcile these findings? Enter the pathologists. As I mentioned above, my first year pathology professor in med school insisted that pathologists were the only doctors who really understood heart disease, because they actually did the autopsies and examined the coronary arteries under microscopes.
And this brings me to my point. The only way Insight #1 and Insight #2 can be correct is if atherosclerosis takes a long time to develop. Any guesses as to what the greatest single risk factor is for heart disease? Smoking? Nope. High blood pressure? Nope. The wickedly deadly particle I have yet to write the most deserving post on, Lp(a)? Nope. LDL-P or apoB? Nope. LDL-C? I thought I told you to never say that again. CRP? Nope. None of these things. It’s age. Age trumps everything. In this sense, atherosclerosis is an “integral” disease (in the calculus sense of the word)—meaning it’s a disease of compounding injuries, as I painstakingly went through above. Age = persistent exposure to LDL-P/apoB.
Just like wealth is compounded in a highly non-linear way, so too is illness and no disease to my knowledge does so more clearly than atherosclerosis. So the jugular question is when do we need to start treating patients? That is a question I can’t answer for you. Not because I don’t have a point of view, which I most certainly do, but because it comes down to risk tolerance. I can no more impart my world view of this problem on you (though the answer seems painfully obvious to me) as I can my world view of how to raise kids or combat ISIS. But I do hope to leave you with a clear picture, at least of the disease process.
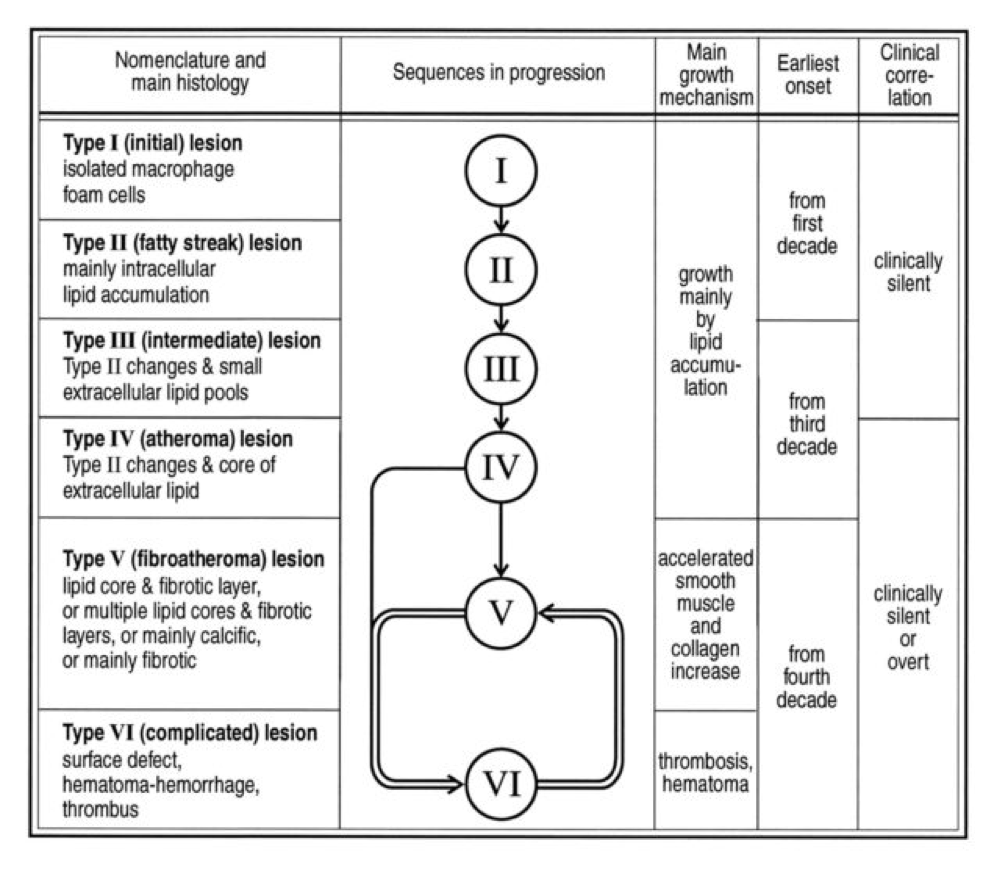
Perhaps the greatest insights into the pathogenesis of atherosclerosis, especially as they pertain to age, comes via autopsies of two variants: those of people known to die of some cause other than heart disease and those known or suspected of cardiac death. The table, below, taken from this paper, summarizes the six stages of atherosclerosis. Stary’s stages are identical, except that he further divides the sixth of these stages into three stages, for a total of eight stages, but the points remain the same. The points being, of course:
- In the first decade of life fatty streaks are being formed. That’s right, before the age of 10.
- Atheromas are present by the time most people are in their 20’s.
- By the time you are in your 30’s you are quite likely to have fibroatheroma formation.
- The vast majority of atherosclerosis-initiating sterols get into the artery as “passengers” in apoB-containing particles most of which (90%) are LDL particles.
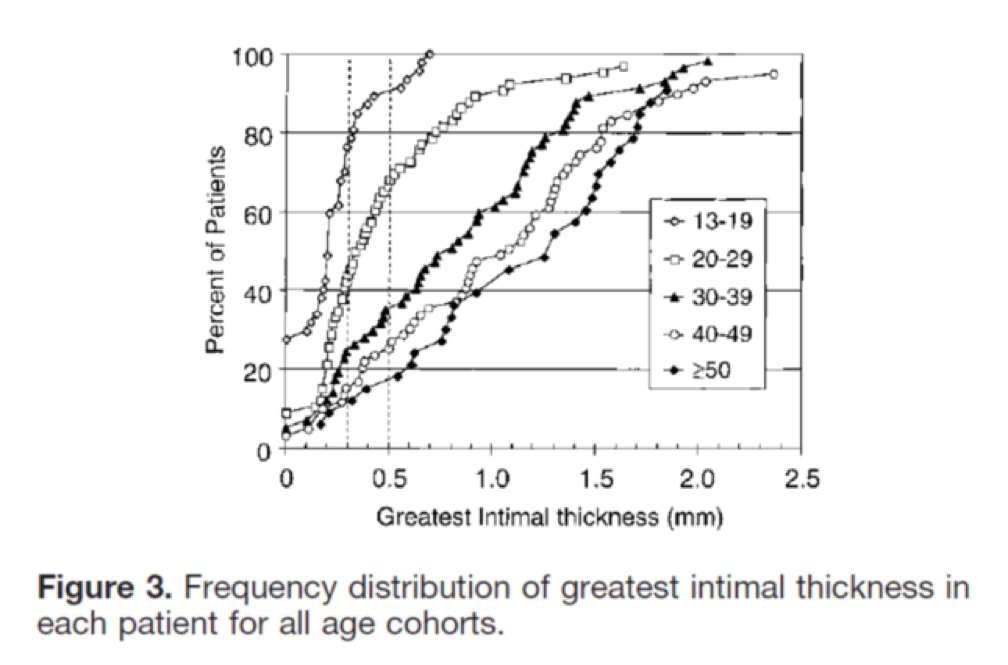
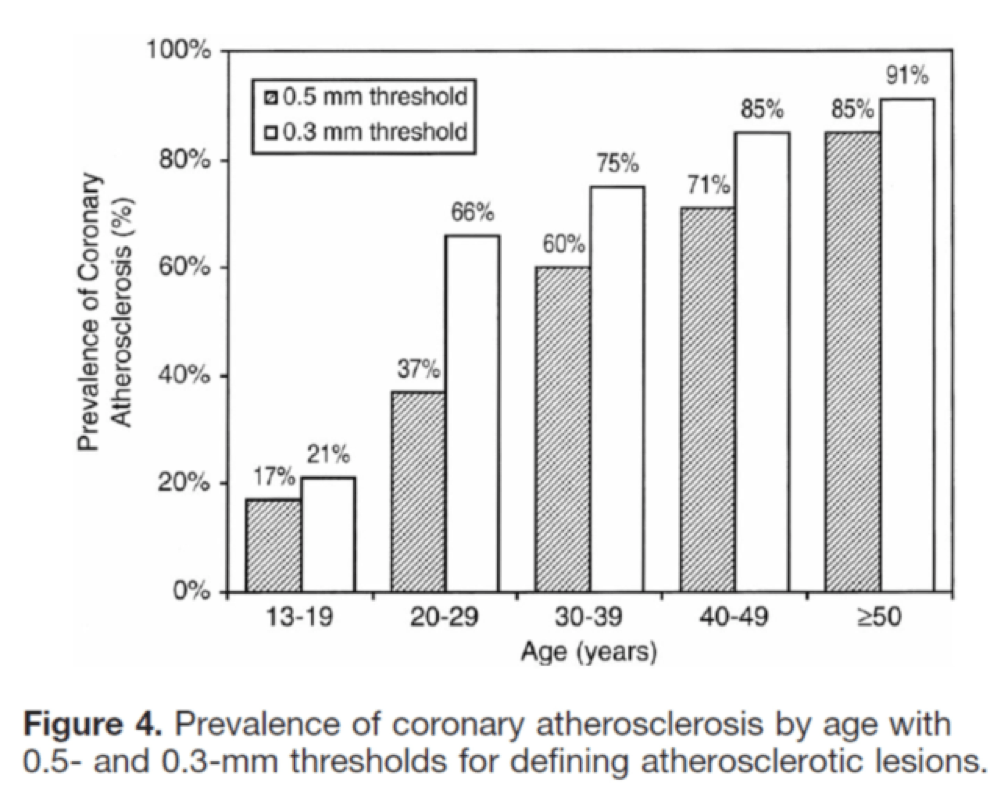
I’ll close with another interesting study, published in Circulation in 2001. The title of the study—High Prevalence of Coronary Atherosclerosis in Asymptomatic Teenagers and Young Adults—pretty much tells you what they found. The authors performed intravascular ultrasound (IVUS) on 262 heart transplant recipients about a month following their transplant. Before going any further, it’s worth pointing out that IVUS is not as sensitive as pathologic sectioning so this study is likely underestimating the degree of disease present in the hearts (but obviously one can’t section the coronary arteries of donor hearts). By doing the IVUS on the recipients so soon after they received their transplant the authors were able to study the hearts of the donors, many of whom were quite young.
The figures below, both taken from the paper, show the frequency distribution and prevalence of intimal thickening for each age cohort of donors. Consistent with the pathology studies, which actually cut open the arteries and examined them histologically, the IVUS study found a similar trend. Namely, atherosclerosis is starting much sooner than previously recognized. In this series of 262 heart donors, one out of six hearts donated by a teenager was found to have clinically measurable atherosclerosis. The authors conclude: “These findings suggest the need for intensive efforts at coronary disease prevention in young adults.”
Final thoughts
I was 35 years old, the year my daughter was born, when I first confronted my own inevitable demise which, based on my family history, was likely to be a cardiovascular one. I’m sure I’d be better off if I had that epiphany when I was 25 or possibly 15, but I’m glad it happened when it did. Today, I manage all modifiable risk factors to the level of my risk appetite and interpretation of the most nuanced scientific literature on cardiovascular disease. Does this mean I won’t succumb to heart disease? Of course not. But this is a stochastic game and the objective of the game is to increase the odds in your favor while delaying the onset of bad outcomes. It’s up to each person, and his or her doctor if necessary, to determine how aggressively he or she wants to confront the inevitable—we all have atherosclerosis at some level.
Addendum
Let me add another thought that may address some concerns.
Clearly there are factors here we (at least I) do not understand. Why do some people just stop with fatty streaks and stay that way for decades, while others progress to clinically significant heart disease? I’m sure if one normalizes for LDL-P, Lp(a)-P, and even particle size, the answer will still elude us (me). I think what people are missing here is that there is a difference between what we can infer from population-based (i.e., heterogeneous) statistics–clinical trials with drugs, epidemiology, ecology, hundreds of autopsies–and what happens at the individual level. Furthermore, the only probabilities that matter when it comes to an individual are “0” and “1”–in other words we go from the most heterogeneous to the most homogeneous.
I’m reminded of an analogy Allan Sniderman shared with me: If you’re a parent, with kids in your home, and want to know the probability that your child (or any child in your home) will die from an accidental gun shot wound, you can look at the stats, which are readily available. They will say the risk is actually pretty low–which it is on average. But what if we now adjust the information (in the Bayesian sense of the word) and note that you have a loaded .45 under your pillow (you know, for home protection). Now what happens to the risk? Of course it goes up dramatically. Why? Not just because we have “more” information–we actually have very specific “new” information. We have information about the causative agent, the gun. LDL-P and Lp(a)-P are the causative agents, and while other forces matter (e.g., inflammatory response), there is no getting around the fact that high LDL-P and Lp(a)-P are stacking the odds against you. The gun won’t pull the trigger on its own, but it’s still the causative agent. People are too quick to confuse “necessary and sufficient” with “necessary but not sufficient”–in both cases reducing the “necessary” component helps.
While I do not doubt that some people with high LDL-P and Lp(a)-P may be immune to the typical “rules” of atherosclerosis, we don’t have good enough tools to predict this, especially non-invasively. Ergo, the risk of doing something to lower these modifiable risks, even if that means taking drugs, must be weighed against the risk of doing nothing. This last point is often dismissed.
Most kids in homes with loaded guns will not die from those guns, but to ignore the loaded gun in that setting is crazy, in my opinion.
I’m going to remove the gun and put the odds back in my favor.
Image credit: Dr. Edwin P. Ewing, Jr. Gross pathology of atherosclerosis, aorta. Autopsy specimen of aorta has been opened lengthwise to reveal luminal surface studded with lesions of atherosclerosis.






“Gerald, I don’t disagree at all that flow characteristics matter (I used to be a mechanical engineer and fluid dynamics was my passion), but I’m still waiting for a better idea to reduce the risk of CHD/CVD than reducing LDL-P/Lp(a)-P and improving the metabolic milieu (e.g., reducing hyperinsulinemia and hyerglycemia). Why does it have to be one or the other? This post focuses on the former. God knows I’ve written plenty about the latter.”
I’d be interested to hear your take on how you reconcile those two viewpoints with this old post from Stephan Guyenet looking at the difference between CVD rates in populations differing in geography and environment, but not genetics:
https://wholehealthsource.blogspot.com/2009/08/ischemic-heart-attacks-disease-of.html
It’s pretty clearly an environmental cause. If it was due to the shape of our circulatory system or carbs, you wouldn’t see this dramatic a divergence (note the huge difference in CVD rates between Japanese living in Japan and those in the US).
Thanks for the personal reply, like you I have many more questions than answers and anyone who tries to keep up with the enormous literature regarding age related diseases knows that anyone claiming truth is a fraudster as “our knowledge only leads to deeper levels of confusion”.
Just a little about me; 66 yr old retired anesthetist/intensivist, read Yudkin in 1967 and have avoided sugar ever since. Bought into the fat hypothesis and also the fiber hypothesis after attending a Denis Burkett lecture in 1971. Always athletic BMI between 20 and 22 and still biking and jogging now. Adopted a typical non focused healthy eating strategy for best part of 30 years and BMI edged up to 24. Read Taubes’ titanic work in 2007 and became much more focused on avoiding all the high glycemic carbs and dropped 20 lbs in 6 weeks: 6 ft and 174 lbs for the last 9 years on a low carb high fiber diet otherwise unrestricted, no calorie counting. Age 64 suddenly developed what I suspected might be stable angina, continued to exercise for 2 years with no change in onset of symptoms. FInally age 65 stress test positive; angiogram isolated CTO right coronary distal to CMFLX and after a complex retrograde canulation of the RCA via the LCA got a couple of stents and am totally angina free and back to HIIT on my favorite hill in the park at The Quarry, Shrewsbury, UK. The figures you show of the near universal finding of some degree of atherosclerosis at age
So I have a personal interest in seeking the ‘truth’ of these matters. An immediate question is the relevance of atorvastatin. In my case I took 40 mg for 11 months and I am 3 weeks into a personal trial stopping the statin and have a feeling?? that exercise recovery muscle aches have improved.
Questions:
What is the evidence that LDL particles penetrate the endothelium of arteries and are the all important cause of plaque. LDL may penetrate endothelium to deliver its energy substrate to cells as a normal physiological function?.
Foam cells are phagocytes engorged with fat that fail to depart the intima and become necrotic leading to fatty streaks and the beginning of plaque formation,
This only happens in certain arterial locations where there is increased endothelial apoptosis. These apoptotic cells are cleared and recycled in a normal fashion by immune cell phagocytosis.
Any situation that may lead to excessive apoptosis and/or less than optimal clearance by the immune cells could conceivably overload the system where necrotic immune cells (foam cells) would start plaque formation process. For instance cholesterol build up in chronic otitis media (cholesteatoma) is residue from insufficiently cleared necrotic immune cells.
Interestingly, with diabetes plaque formation is much more diffuse rather than localized.
Endothelial cells and immune cells have insulin receptors. Macrophage activation and endothelial function are influenced by insulin and disturbed with hyperinsulinemia and regular postprandial hyperglycemia.
Autophagy is necessary for phagocytosis is suppressed by insulin through mTOR activation.
Lipoprotein B (LDL) synthesis by the liver is stimulated by insulin, the weak association of LDL with CAD events may be, even more likely confounding than causative. JUPITER study suggests that the protective effect of statins was more likely due to their anti-inflammatory side effects as it was not related to LDL levels. In fact the benefits of statins were only seen in patients with raised CRP.
Clearly CAD is complex and my main point in all of this is to expose a red herring for the damage it can do and it’s looking more and more like LDL is a red herring, meanwhile huge resources of money and attention are being diverted to controlling it.
Interesting historical studies on Japanese in Tokyo and California, maybe the difference was caused by the greater accessibility to rice in those living the good life in California?
Much appreciate the chance to do some thinking here, hope to follow your blogs from now on, Cheers
Gerry
Gerry, see a more recent analysis of JUPITER (Circulation. 2015;132:2220-2229). Lots of good questions, but it’s all I can do to write the posts.
To provide an answer to some of Gerald’s questions, I posted this as a comment at Hyperlipid’s site earlier. To be clear, I eat low-carb myself, but I don’t think that’s the root cause of our issues with the metabolic syndrome any more.
To your comment about endothelial apoptosis, the cause of that is pretty well-determined, you just need to find the right term to search for. oxLDL is a better guide to CVD than other things, and it’s pretty clear what oxidizes LDL, it’s the same thing that causes oxidative damage throughout the rest of the body. And it’s not glucose. That term is below.
“Whilst relatively low doses of HNE can orchestrate cell signaling events, higher concentrations of HNE appear to modify a further set of target proteins, inhibiting or dysregulating previously functional cellular processes and organelle functions. In particular, endothelial cells, which form the primary vascular interface for potentially oxidized circulating components, appear to be highly susceptible to HNE induced damage [10,85]. HNE can exert a range of pathophysiological effects, including interfering with the synthesis and release of vasoactive mediators, breakdown of the endothelial barrier function and inducing a pro-inflammatory phenotype within the vessel wall.”
It also impairs NO, but that’s not all!
“Elevated plasma levels of HNE also damage endothelial barrier function [10,87] due to impaired cell–cell communication and inhibition of membrane associated enzymes [85].”
There’s also this:
“Cholesterol consumption reportedly increases HNE synthesis [11], with HNE inducing low-density lipoprotein oxidation and increased uptake by macrophages [12], and HNE accumulation within atherosclerotic plaques [13].
“Effects of 4-hydroxynonenal on vascular endothelial and smooth muscle cell redox signaling and function in health and disease”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3757694/
Another paper sums it up thus:
“Both HNE and oxysterols then appear to be candidates for a primary role in the progression of the atherosclerotic process.”
“4-Hydroxynonenal and cholesterol oxidation products in atherosclerosis”
https://onlinelibrary.wiley.com/doi/10.1002/mnfr.200500090/abstract
But even that’s not all!
“Among the factors known to accumulate with aging, advanced lipid peroxidation end products (ALEs) are a hallmark of oxidative stress-associated diseases such as atherosclerosis. Aldehydes generated from the peroxidation of polyunsaturated fatty acids (PUFA), (4-hydroxynonenal, malondialdehyde, acrolein), form adducts on cellular proteins, leading to a progressive protein dysfunction with consequences in the pathophysiology of vascular aging. The contribution of these aldehydes to ECM modification is not known. This study was carried out to investigate whether aldehyde-adducts are detected in the intima and media in human aorta, whether their level is increased in vascular aging, and whether elastin fibers are a target of aldehyde-adduct formation. Immunohistological and confocal immunofluorescence studies indicate that 4-HNE-histidine-adducts accumulate in an age-related manner in the intima, media and adventitia layers of human aortas, and are mainly expressed in smooth muscle cells….”
“The accumulation of 4-HNE-adducts is very high in the intimal aorta, mainly in older patients with high atherosclerosis grade. These data were expected since oxidized LDL and lipids accumulate in the intima in the early lesions and in the lipid core of advanced atherosclerotic lesions [23,24]. These data confirm that 4-HNE is a main marker of oxidative stress and LDL oxidation which could contribute to the evolution of the lesions via its ability to modify proteins and generate cell dysfunction. 4-HNE expression was also increased in the adventitia of the elderly, probably associated with the vasa vasorum, which are involved in the supplying of nutrients and oxygen to atherosclerotic lesions, and the development of angiogenesis in the atherosclerotic plaque [32,42]. The recently reported angiogenic effect of 4-HNE suggests a role for this aldehyde in the development of vasa vasorum and microcapillaries in atherosclerotic plaque [35,43].”
“Elastin aging and lipid oxidation products in human aorta”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4309857/
Hi Peter,
When thinking of endothelial cell damage I always think of hyperhomocysteinemia. Why no mentiona, studies still too ambiguous?
If reversal was possible, what could the mechanism theoretically be and what would impact it most? Here some wild guesses: VLCD (to become fat adapted), endurance (higher avg blood flow) or interval taining daily (blood pressure variation), hyperbaric oxygen treatment (to increase ROS and destroy those foams), extended fasts (like 10-14d) … any guesses?
It probably contributes, but I omitted many things that damage the endothelium (smoking, HTN) much more than homocysteine.
Your contributions are priceless in helping people take charge of their health. “Start with keeping LDL-P as low as possible.” I hope you will address how to do this. Since adopting LCHF all of my cardiac bio markers have improved (as well as several chronic health conditions that have disappeared) with the exception of small LDLp, which is close to 2000. The standard advice to opt for plant oils in lieu of animal fats will rob Peter to pay Paul. I am not a candidate for statins due to risk of heart failure, which killed my mother, and their benefit is dubious. Will increasing vitamin D sulphate via sun exposure improve LDLp? What else is there?
I am not sure if vit D will reduce LDL-P, but if it does I would expect the effect to be very small.
Thanks for another brilliant post that’s also easy-to-understand by non-medical people…
I just want to know, maybe based on your own observation, that a low LDL-C or VLDL-C can also mean low LDL-P? or to put it in another perspective, would it be possible to have high LDL-P though LDL-C is low?
Also, correct me if I am wrong, I still remember in one of your articles about apoB/cholesterol that the consumption of sugar is directly concordant with apoB or LDL-C. My own experience on a LCHF for almost 4 years showed that my LDL-C decreases – would this be safe to assume that my LDL-P is also low?
I don’t have access to NMR testing for LDL-P at the moment, it’s not available in my country that’s why seeing my LDL-C low maybe a good sign (or not based on your own thoughts).
Thanks again and more power!
Please see series on cholesterol.
I’m not sure whether or not you have already read/heard of Uffe Ravnskov’s theory of atherosclerosis; but I think that you might find it interesting.
https://www.spacedoc.com/articles/uffe-ravnskov-the-real-cause-of-heart-disease
Great article Dr Attia! Even though this blog might not be the forum, I’d love to see your thoughts on the p4p king of all time! Unless, of course, you choose someone other than Ray Robinson, because then you’re just flat out wrong… Seriously though, I’d love reading that!
There are two separate issues: who is the P4P king and which fight was the greatest performance of all time. I can make a case for all of these guys being the greatest of all time (and keep in mind, we we robbed of Ali’s best 3 years), but I lean to Ali vs. Williams in Nov ’66 as the greatest performance of all time. Jim Jacobs noted on the film that Ali’s hand and foot speed–on that night at least–exceeded that of Robinson.
Peter,
Another great article. Thanks for this post. I’ve followed your blog and Dr. Dayspring’s work for years and have benefited personally and professionally. Keep up the good work. As to HDL-C’s role in the disease process, I’d love to be able to measure HDL-TG. That might show some of the function of HDL particles. Those particles overloaded by TG from excessive carbohydrate intake and hyperinsulinemic states probably can’t lipidate as well thereby leaving the macrophages engorged. The increased oxidation and inflammation driven by the hyperinsulinemia only worsens the disease process.
Thanks again,
Mark
Mark, these assays are being worked at this moment. In fact, LDL-TG and HDL-TG may prove to be great functional tests, as you note (certainly better than cholesterol content and probably accretively with particle size and number).
Any known effects on apoB/Lp(a)-P/LDL-P by CETP inhibitors? I’d be pretty telling….
CETP inhibitors were thought to improve outcomes by raising HDL-C (when it was believed such a thing was helpful). I believe their first trial was in combination with atorvastatin.
Trying to summarize your article: no oxLDL, no problem –> reduce oxidation + reduce LDL. That simple? Take statins and Anti-Oxidant? (with Vitamin E at least that failed at least due to some specific effects of Vit E)
Quick check on new oxLDL studies and learning that it is a gradual oxidation process from mildly (=no immune response) to heavily oxidized, some suggest it is still controversial, for example:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4612790/table/tbl01/ (pathogenic, neutral, protective results)
Disappointing really, has been studied for 20-30 years and still we lack detailed level understanding…
Part of the trouble, Andre, is that (to my counting) there are still 3 assays out there to measure oxLDL in some capacity or another. So this tool is still in its infancy.
Agree on the oxLDL, too many sites on the LDL that can be oxidized with different effects contribute to the problem.
What is your view on PAI, TG, C-Peptide and HDL vs ApoB and Insulin?
https://www.thefatemperor.com/blog/2015/4/25/lchf-motherlode-paper-ldlp-my-butt-insulin-and-c-peptide-rules-atheroscleosis
This makes me want to write another post to clarify all the confusion around biomarkers. Folks need to understand that all cardiac biomarkers need to be evaluated in a 2×2 matrix: X-axis is causal vs. not causal (e.g., LDL-P is causal; CRP is not); Y-axis is treatable vs. not treatable (e.g., LDL-P is treatable; Lp(a)-P is not*). There is so much mindless rambling about this biomarker versus that one, but folks are missing the big picture. I’m a framework guy, frankly, because it forces a modicum of discipline. Perhaps I’ll get to this in the next year. I certainly have a lot to say! (* Lp(a)-P has historically not been treatable, but PCSK9i are lowering by as much as 30% and I’ve seen >50% in one patient, and soon anti-sense drugs will, hopefully, render Lp(a) obsolete).
“LDL-P is causal”
As I know you know, apart from extreme cases establishing causation in biology is no easy task. The history of biology and medicine is littered with causal claims that turned out to be either false or vastly oversimplified.
Perhaps I’m missing something, but I’ve yet to see anything like evidence that truly establishes causality for LDL-P in CVD. Significant associations with possible direct causal links, yes, but it takes so much more than that. At some point it would be instructive to hear why you are convinced enough about LDL-P to use non-benign pharmaceuticals to lower it.
And even beyond that (If I understand correctly) to screen seemingly healthy people for LDL-P and treat at least some of those who test positive. Even beyond the steep challenge of really knowing a “risk factor” is causal, the history of screening apparently healthy populations is not very encouraging. The more great sounding ideas such as screening PSA tests and mammography are carefully studied the less enticing their benefit/risk ratio appears.
PS: In the case of illnesses that will be very rare in the screened population, as we know Bayes’s Theorem requires almost 100% specific tests to avoid harms exceeding benefits. We don’t generally have tests like that, one of the fundamental problems with screening. I recognize that if you believe CVD is *not* rare in the screened population Bayes’s Theorem is more forgiving of imperfect tests. But it’s still a steep challenge, to me at least, to have confidence that benefits will outweigh harms. Of course you could say the same thing about not screening, but under conditions of extreme uncertainty I prefer the razor of nonintervention. I realize that sometimes that will be a mistake.
I think the best case for LDL-P being causal is made from a few combined observations:
1. Heterozygous FH patients
2. The folks with nonsense PCSK9 mutations. This was described 10 years ago in NEJM (see Fig 1): https://www.nejm.org/doi/full/10.1056/NEJMoa054013#t=article
3. The K-M curves for MESA and Framingham comparing LDL-P vs. LDL-C
4. LDL-C genes like HMGCoA and others are linked with atherosclerosis causality in Mendelian randomization trials. This is as close to causality as you get without doing an experiment which, obviously, can’t be done in this case.
Furthermore, the only sine qua non for atherosclerosis is sterols in the artery wall. They do not come from de novo synthesis but rather LDL particle trafficking through the endothelium or vasa vasorum.
Bill, I’m not here to convince anyone to do anything, including my own patients, let alone a bunch of complete strangers reading my blog.
But I hope people concerned with the risks of taking drugs when necessary to lower LDL-P (or Lp(a)-P) also consider the risk of NOT doing so.
Before writing anything, please also have a look at Rhonda interviewing Dr. Satchin Panda on Time-Restricted Feeding and Its Effects on Obesity, Muscle Mass & Heart Heal 😉
https://www.youtube.com/watch?v=-R-eqJDQ2nU
conforims the benefits of intermittent fasting (12-16h), Mitochondria optimization, different clocks we have and which need to be in synch (liver/food, light, activity)
Peter, thanks for taking the time to answer this.
“I’m not here to convince anyone to do anything, including my own patients, let alone a bunch of complete strangers reading my blog.”
Understood. I only wanted to appreciate your thinking on this better. I’ve always found that smart people with different views can be my best teachers :-).
evacetrapib — Participants taking the drug saw their LDL levels fall to an average of 55 milligrams per deciliter from 84. Their HDL levels rose to an average of 104 milligram per deciliter from 46. Stopped Stage 3 trial. No benefit.
Add it the graveyard.
Dear Peter Attia
Great post.
Just wondering, instead of apoB INTERHEART found apoB/apoA a more solid indicator for MI
Regardless of the absotule apoB level ?
Any thoughts ? Or studies.
Best Mauricio Trambaioli
I’m not really sure what to make of that, but if I recall apoB/apoA1 was even higher HR than smoking vs. not, which seems odd to me. Smoking is generally a higher risk than apoB.
Peter-
Your ldl-p / gun analogy is interesting. Not to be too literal, but it got me thinking- a loaded gun under your pillow in a war zone without any children around could be smart. Understanding the ldl-p link with heart disease (using poor overall societal health status including SAD diet, inactivity, stress, etc) is there any physiological benefit of elevated ldl-p you are aware of, particularly in the absence of obvious known risk factors? For it to be purely negative seems a strange biological program-?
Probably benefits to high cholesterol, though not sure there are benefits to high LDL-P. If high HDL-P can do most of the trafficking, then a great state of life might be modest-to-high cholesterol with lots of apoA and few apoB.
Dr. Dayspring blew my mind when he tweeted that there is no need for LDL at all – the body is fine at 0! I got the feeling he doesn’t think there are any benefits…
Well, some of the people with nonsnese PCSK9 mutations have LDL-C levels of 10-20 mg/dL (!!!) and seem to do just fine in addition to being seemingly immune to atherosclerosis.
Dr Peter,
A great article ..further to your series on Cholesterol.
But, to put everything squarely on ” AGE ” …..To quote you..
“Smoking? Nope. High blood pressure? Nope. The wickedly deadly particle I have yet to write the most deserving post on, Lp(a)? Nope. LDL-P or apoB? Nope. LDL-C? I thought I told you to never say that again. CRP? Nope. None of these things. ” None of this may be, is the greatest single risk factor.. You may be right there….
But is it not true that ……CVD is the result of a cumulative effect of multiple risk factors,.
……the body is a drum with multiple abuse inputs leading to overflow (VHD/CVD, Cancer).
What about the importance of food and lifestyle in ” trying to remove the gun and put the odds back in our favor.”
My sincere respects to you for a great article.
You missed my point, but I’m too lazy to write it out more clearly. Hopefully someone else does so for me. You’re confusing the greatest absolute risk (which happens to NOT be modifiable) with all forms of modifiable risk.
Hi, dr.Peter 😉
In my case I had a heart stroke at 37 (stent in my left coronary).. The diagnosis was a hypercholesterolemia..
I had a stroke when practice spearfishing (apnea).
After stent implantation they prescribed me to take 20mg statin drug. My total cholesterol level is 8 mmol/l.. with statins this went down to 4.3 but I had several pain in my legs and chest so I leaved them.. I also tryed with LCHF food.. with LCHF my tryglicerides went down from 1,3 to 0,6 mmol/l , LDL went from 4 to 6 and HDL from 1 – 1.5 mmol/l.. (I don’t know if this if good aproach)..
After one years I went to my doctor (cardio) and he prescribed me a new medicine “Repatha”.. It’s now second week I’am using it (without any side effect for now)..
I’am little confused because some say that LCHF is danger for atherosclerosis some say that isn’t…
I practice sport 3-4 times a week (running, cardio trennings, spearfishing), I don’t dink and I never smoked..
I’am 181 cm height and weight 82 kg… (same weight last 20 years)..
Here is my complete cholesterol levels from 2014 til now…
https://s5.postimg.org/4q77vk9zb/screenshot_195.jpg
What do you think about hypercholesterolemia? (I have also a doughter 8 years old with high level of cholesterol) (also my father and mother)..
thank you for any info.
Peter you likely have a genetic condition and should see a lipid specialist to confirm this. If you do have a genetic condition, it will important for your kids to be checked also.
Checked the reanalysis of JUPITER Circulation. 2015;132:2220-2229.
In placebo-allocated participants, baseline LDL-C was not associated with CVD (adjusted hazard ratio [HR] per SD, 1.03; 95% confidence interval [CI], 0.88–1.21). In contrast, associations with CVD events were observed for baseline non–high-density lipoprotein (HDL) cholesterol (HR, 1.18; 95% CI, 1.01–1.38), apolipoprotein B (HR, 1.28; 95% CI, 1.11–1.48), and ion mobility–measured non-HDL particles (HR, 1.19; 95% CI, 1.05–1.35) and LDL particles (HR, 1.21; 95% CI, 1.07–1.37).
Worst Hazard Ratio was for Apo B=1.28 while LDLp = 1.21. While the association my be statistically significant it is so marginal to be hardly relevant to actual cause and much more likely to be peripheral or downstream effect of other causes. For instance HgbA1C between 6.5-7% in EPIC-Norfolk was associated with HR 3.0 while higher HgbA1C HR 7.0. Little old LDL comes in 1.3 at best and 1.03 (NS) in 2 the above placebo arm. HR of smoking and lung cancer is 13-20, and that’s the sort of power you need to establish cause. Are we guilty of sleeping at the watch while the safe is being burgled?
I don’t think the HRs here establish cause. Agree that HR below 5-10 is not helpful for that reason. The reason (in my opinion) HRs are lousy for diseases like heart disease is that the condition is so prevalent. And, as pointed out, apoB is only necessary, but not sufficient to initiate the disease.
Hi Peter !
Thanks for your reply on Lp(a) – agreed there’s a lot more going on here – with Lp(a) kinda carried along for the ride perhaps. 🙂 Multi-factor fun I guess.
Anyway, on to more serious matters. I thought a little at the weekend while preparing slides for an upcoming talk I’m giving (to big group of Paramedics). I drifted down the ApoB hole whilst doing a simplified Root Cause Diagram (to help them understand the basics of CHD driving mechanisms).
My thoughts on ApoB as an independent driver surfaced as I drafted. There are lots of comments already on this post (unfortunately they are chronologically ordered from the bottom one up – Squarespace, eh?). Anyway, would be interested in your thoughts – although I will understand if you are too busy to run through the content…
https://www.thefatemperor.com/blog/2016/7/9/atherosclerosis-high-level-root-cause-the-apobldlp-question
Thanks as always for your assessments
Best
Ivor
http://www.thefatemperor.com
Great article.. what do you think of the predictive value of calcium scoring?
Probably adds some value in young patients (i.e., ID those at high risk), but if anything, folks on statins probably have better outcomes and calcification increases (plaque stabilization).
Thank you for the article, Peter!
I was recently researching autophagy, it seems autophagy (or rather lack of it) is involved in atherosclerosis too. Relevant studies:
– mTOR Enhances Foam Cell Formation by Suppressing the Autophagy Pathway
https://online.liebertpub.com/doi/abs/10.1089/dna.2013.2164?journalCode=dna
– Nicotinate-Curcumin Impedes Foam Cell Formation from THP-1 Cells through Restoring Autophagy Flux
https://journals.plos.org/plosone/article?id=10.1371%2Fjournal.pone.0154820
– Regulation of Lipid Droplet Cholesterol Efflux From Macrophage Foam Cells
https://atvb.ahajournals.org/content/32/3/575.full
Research on autophagy seems relatively new, but logically it makes sense. High levels of insulin from the modern diet & constant snacking (no fasting) reduce autophagy, which likely contributes to atherosclerosis
No doubt mTOR–which is probably the central cellular regulator of autophagy–plays a role in all disease. CHD is no exception.
Peter,
Another great post. Thank you.
I look forward to your post about Lp(a). In that post, it would help me (and perhaps others) if you addressed the implications of Chasman’s findings (Atherosclerosis, April 2009) about the effects of low-dose aspirin on some people with particular polymorphisms of the Lp(a) gene.
I am intrigued by the finding that aspirin seemed to eliminate the increased risk that some people have from high Lp(a) levels. Of course, I wonder what that finding means for others with high Lp(a), when we don’t know other details about our generic make-up.
Thanks again,
Richard
Yes, this would be a great post, Richard. Of course, what makes Lp(a) so “bad” is not only its particle properties, but also its thrombotic properties. Not sure if LpPLA2 helps stratify risk.
Hey Peter,
Just wanted to reach out and say thanks for writing this post. Also thanks for appearing on Tim Ferriss podcast where I was first introduced to you. The 2 awesome youtube videos; “Readdressing Dietary Guidelines”, and “An Advantaged Metabolic Stage.” have been a huge help to me and my personal improvement journey. Keep fighting the good fight. 🙂
Chris.
Thank you, Chris. Glad you find them helpful.
Peter,
Your contenders for greatest boxers of all time seems rather American, and still missing Joe Gans! I would want to nominate Bob Fitzsimmons and Jim Driscoll, in the latter case emphasising ‘boxer’ rather than ‘puncher’. Obviously this is a futile discussion, but fun!
As someone with next to no understanding of biology or chemistry I should perhaps have given this post a miss but having now read it three times I think I may have caught the gist of it.
I think it is worth pointing out that if you are right about the efficacy of medication in increasing one’s chances of prolonging life that does require the caveat that the longer life may come at the cost of impaired physical and mental functioning. I was on statins for about 10 years and the arthritis and increasingly foggy brain function I had were blamed by my doctor on inevitable consequences of getting old. When I decided, about 5 years ago, to stop taking statins my physical health and brain function returned over time to a remarkable degree. In the case of physical health this is to some extent an objective judgement, not only have the pains diminished significantly but I am stronger than I was 10 years ago (I have gym records to prove it) and my maximum heart rate has increased from the low 160s to 180 last week (I am close to 67 years old). You must also be aware that there are blogs where people who seem to know what they are writing about assert that statistically, in old age, high cholesterol appears to be protective when it comes to heart disease.
It’s all very confusing! But many thanks for taking the time to let us share your research and expertise, it is much appreciated.