If I held a crystal ball 10 years ago, I’m not sure I would’ve believed it if it showed me the increased interest in the ketogenic diet would look like the figure below. That’s 2 logs, folks.
Admittedly, I started my journey on this path in 2009, with a deep dive into ketosis in the Spring of 2011, but it seemed so obscure! (For a timeline of what I did, I think I covered it somewhere in this talk…yes I’m too lazy to actually confirm this by skimming through it.) All told I spent approximately 3 years in the strictest state of nutritional ketosis (NK) with one very memorable deviation when I had 6 or 7 full-sized and upsettingly decadent desserts circa September 2013. I believe the diet helped me transition from metabolic syndrome to metabolic health and I certainly thought it could benefit other people. This nutritional state could gain some steam, I thought.
I was well aware of the dearth of mainstream knowledge of NK, and particularly the conflation of NK with diabetic ketoacidosis (DKA), a pathologic state that results from the complete or near absence of insulin, which is what prompted my writing and desire to share my journey. And I was once in the wanker category of folks who spoke with “authority” about ketosis, despite knowing somewhere between zero and nothing on the topic. I remember exactly where I was sitting in a clinic at Johns Hopkins in 2002 during my residency explaining to (admonishing, really) a patient who was on the Atkins diet how harmful it was because of DKA. Not only that, the ketogenic diet could be seen as the antithesis of a “healthy” diet by conventional standards. I could see how this was a difficult proposition for many to acknowledge.
The beautiful part of good science is its self-correcting nature. The ugly part is this self-correcting nature often moves at a glacial pace—and it’s not linear. We often view history century-by-century and see what amounts to continual progress in medicine. But we live our lives—and consume information—day-by-day, exposed to the peaks and valleys of medical wisdom.
Looking back on my earlier posts on ketosis—and explaining what I eat, for example—makes me both chuckle and cringe. I remember how bizarre the diet seemed to many readers and the general public at the time. I also remember digging into the literature and learning, for example, that my alma mater, Johns Hopkins had been using the ketogenic diet to treat pediatric epilepsy for almost a century…and being so embarrassed about admonishing that patient I saw in my residency.
Since then, it’s safe to say I dove down the rabbit hole. The more I learned, the more I grew tired of reading so much misinformation on the topic. While there are more thoughtful people and articles on the subject of ketosis these days (e.g., here’s a thoughtful video on ketosis and ketogenic diets from one of my most important ketosis mentors, Steve Phinney, a co-founder of Virta Health1Disclosure: I’m an investor in, and advisor to, Virta Health.), there are still pieces like the one Vox published this month, that doesn’t exactly do the topic justice.
Like many variables in diet, health, and disease, it behooves us to look beyond the bumper sticker explanation. I want to highlight a couple of posts I wrote, to attempt to provide a little more nuance and understanding to the subject: “Ketosis — advantaged or misunderstood state?” Parts I and II. Part I follows below. I’m hoping to write more on the topic in the not-too-distant future since there’s been a number of intriguing papers published recently (certainly since 2012). But I also wanted to bring these back into focus in light of the information I’m seeing more of on the interwebz. (You can also visit the Ketosis section of the site to view more articles on the subject.)
Because I know people will ask, I have not been on a ketogenic diet “regularly” since about mid- to late-2014. The reasons are too nuanced to describe here, but my deviation is not because I lost confidence in its efficacy. With nearly a decade of clinical experience, I can safely say I was an outlier (in the best sense) with respect to my physiology and response. I was leaner, and more mentally and physically fit during this three year period than during any other period of time as an adult, and my biomarkers were as good as they had ever been. I’ve also seen the benefit of ketogenic diets first-hand on my patients and my own sister, a remarkable story I hope to share one day. But I’ve also been humbled by my inability to explain why some people have suboptimal or even negative responses to NK. I would say, all things considered, my knowledge of ketosis is greater today than when I was writing about it voraciously, but my confidence in my understanding of it, might actually be lower. As the saying goes, the further one goes from shore, the deeper the water gets.
—P.A., April 2018
§
(Part I: originally posted November 26, 2012)
In part I of this post I will see to it (assuming you read it) that you’ll know more about ketosis than just about anyone, including your doctor or the majority of “experts” out there writing about this topic.
Before we begin, a disclaimer in order: If you want to actually understand this topic, you must invest the time and mental energy to do so. You really have to get into the details. Obviously, I love the details and probably read 5 or 6 scientific papers every week on this topic (and others). I don’t expect the casual reader to want to do this, and I view it as my role to synthesize this information and present it to you. But this is not a bumper-sticker issue. I know it’s trendy to make blanket statements – ketosis is “unnatural,” for example, or ketosis is “superior” – but such statements mean nothing if you don’t understand the biochemistry and evolution of our species. So, let’s agree to let the unsubstantiated statements and bumper stickers reside in the world of political debates and opinion-based discussions. For this reason, I’ve deliberately broken this post down and only included this content (i.e., background) for Part I.
What is ketosis?
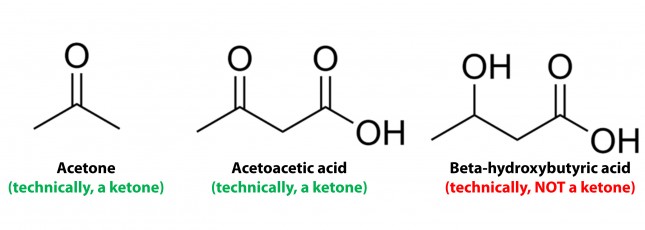
Ketosis is a metabolic state in which the liver produces small organic molecules called ketone bodies at “sufficient” levels, which I’ll expand upon later. First, let’s get the semantics correct. The first confusing thing about ketosis is that ketone bodies are not all – technically — ketones, whose structure is shown below. Technically, the term ketone denotes an organic molecule where a carbon atom, sandwiched between 2 other carbon atoms (denoted by R and R’), is double-bonded to an oxygen atom.
Conversely, the term “ketone bodies” refers to 3 very specific molecules: acetone, acetoacetone (or acetoacetic acid), and beta-hydroxybutyrate (or beta-hydroxybutyric acid), shown below, of which only 2 are technically ketones. (The reason beta-hydroxybutyrate, or B-OHB, is not technically a ketone is that the carbon double-bonded to the oxygen is bonded to an –OH group on one side, technically making B-OHB a carboxylic acid for anyone keeping score.)
Now, back to the real question at hand. Why would our body make these substances? To understand why or when the body would do this requires some understanding of how the body converts stored energy (the food we eat or the energy we store in our body, i.e., fat or glycogen) into phosphate donors. For a refresher on this process, please refer to the video in this post, specifically the section from 2:15 to 13:30.
The ATP issue
As you may recall, about 60% of the energy we expend, say 1,800 kcal/day for someone consuming 3,000 kcal/day in weight balance, is purely devoted to keeping us alive by generating enough ATP (“energy currency”) to do 2 things: allow ion gradients to function and allow muscular relaxation. So, obviously, we can’t tolerate – literally even for one minute – insufficient ATP production. In fact, one of the most potent toxins known to man (cyanide) exerts its effect on this process by inhibiting the electron transport chain which generates the bulk of the ATP our body produces. Even the most transient interruption of this process is fatal.
Take home message #1: No ATP, even for 1 minute, equals no life.
The brain issue
The brain is a particularly greedy organ when it comes to energy requirement. To put this comment in perspective consider the following: though our brain represents only about 2% of our body mass, it accounts for about 20% of our energy expenditure. (In children, by the way, this may be closer to 40-50% of basal metabolic demand.) So, beyond the ATP issue, above, there is a substrate issue with the brain as neurons derive most of their energy from glucose. While there is emerging evidence that neurons can also oxidize fatty acids directly in small amounts and may even prefer lactate (over glucose), these two substrates do not approach the levels of consumption by neurons that glucose does. So, for the purpose of this discussion, let’s just focus on the need of the body to provide glucose to the brain.
You’ll recall, from the point I made above, that my brain requires about 400 to 500 kcal of glucose per day (100 to 120 gm). You’ll also recall (from the video, above) that I can store about 100 to 120 gm of glucose in my liver. While I can store much more in my muscles, (on the order of about 300 to 350 gm), because muscles lack the enzyme glucose-6-phosphatase, glucose stored in muscle as glycogen is unable to re-enter the bloodstream and is meant for the muscle and the muscle alone to use. In other words, muscle glycogen is a stranded asset of glucose in the body to be used only by the muscle.
So, if I’m deprived of a dietary source of glucose, I depend solely on my liver to release glycogen (a process known as hepatic glucose output, or HGO). How long can HGO supply my brain with sufficient glucose? It depends on a few things that impact both the “source” and the “sink” of glucose. Other competing sinks for glucose (e.g., activity level, thermogenic needs) and sources (e.g., glycerol and gluconeogenic amino acid availability) can make a difference for a while. But, in a state of starvation we’ve only got about one to three days before we’re in trouble. If our brain doesn’t get a hold of something else, besides glucose, we will die quite unceremoniously.
Take home message #2: No glucose for 24-72 hours equals the need for something else the brain can use instead (that is not fat or protein, since neurons can’t oxidize fat and the last thing we want to do is start muscle wasting at a geometric rate).
The Krebs Cycle
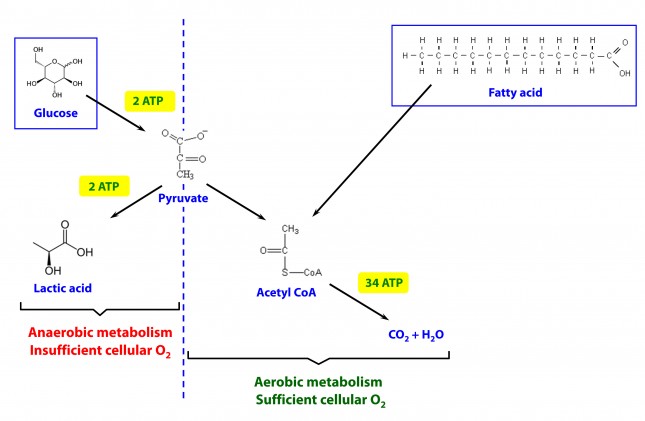
This poses a real evolutionary dilemma. We need an enormous amount of energy just to not die, but the single most important organ in our body (also quite energy hungry in its own right) can’t access the most abundant source of energy in our body (i.e., fat) and is, instead, almost solely dependent on the one macronutrient we can’t store beyond a trivial amount (i.e., glucose). Obviously our species wouldn’t be here today if this were the end of the story. But, to understand how we survived requires one more trip down biochemistry memory lane. In the figure below (also included and described in the video) I gloss over a pretty important detail.
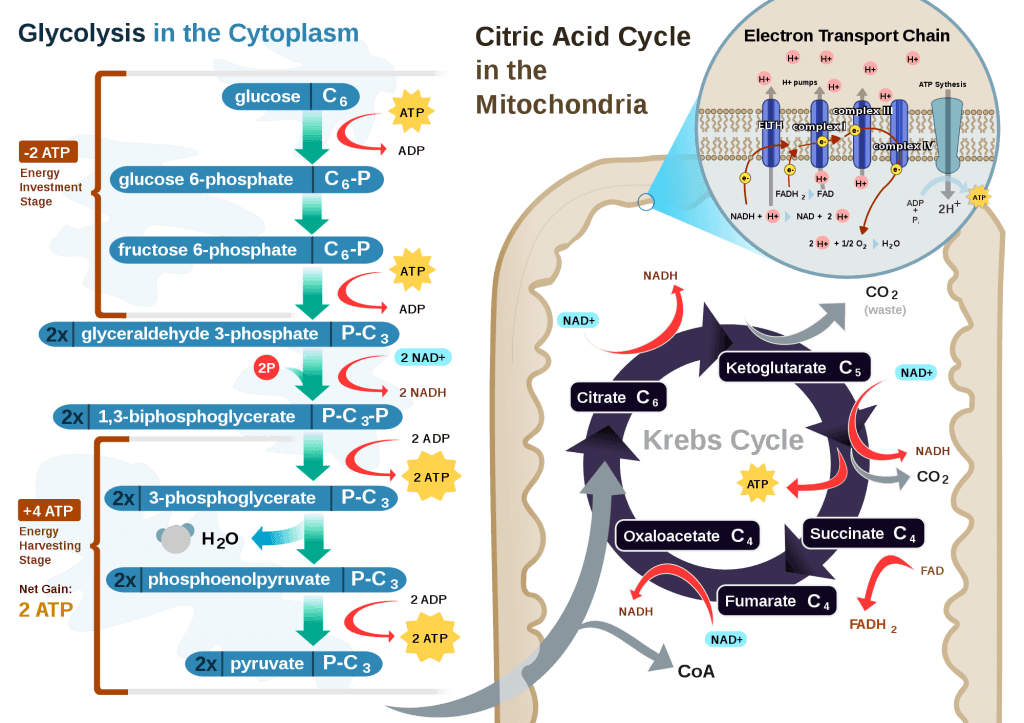
How, exactly, does our body take pyruvate (from glucose) or acetyl CoA (from fat) and generate so much ATP? The answer lies in the beauty of the Krebs Cycle, which feeds into a process called the electron transport chain (or ETC), I alluded to above. Since the adage ‘you can’t get something for nothing’ is as true in biochemistry as it appears to be in life, to get all that ATP (i.e., stored energy in the form of the phosphate bond), we need to give up something. What the ETC does give up, as its name suggests, is electrons. Through a series of redox reactions the ETC trades the stored energy held by electrons going from higher to lower energy states in exchange for the chemical energy stored in the bonds of the third phosphate group on an ATP molecule.
To think of it another way, if you start with stored energy – glucose or fat, for example, which if burned in calorimeter will give off varying amounts of heat – and you’re willing to convert their carbon, hydrogen, and oxygen molecules into another form with less energy – water and carbon dioxide which, if burned, produce very little heat – it’s a fair trade! The ETC is simply the vehicle that allows our body to make the switch.
In a car, by contrast, it’s much simpler. The engine combusts the hydrocarbon (e.g., gasoline) directly and in one flash liberates the heat contained within the hydrogen-carbon and carbon-carbon bonds in exchange for carbon dioxide, water vapor, and a few other things.
If you take a look at the figure, below, you’ll get a sense of the moving pieces involved in this cyclic transfer process. Molecules shuffle back and forth, around the cycle, and kick off spent carbon (carbon dioxide, termed “waste”) and reducing agents (e.g., conversion from NAD+ to NADH) for the ETC.
Where do the ketones come in?
In the absence of acetyl CoA (several ways this can happen, including substrate shortage, as I’m describing here) we evolved a cool trick. Our liver can make – out of fat or protein, though we much prefer to use fat so we can spare our protein and prevent severe muscle wasting – something called beta-hydroxybutyrate, one of the 3 ketone bodies I described above.
B-OHB and acetoacetate (see figure below from this paper by Cahill and Veech, 2003) are produced by the liver from long and medium chain fatty acids and released into the bloodstream.
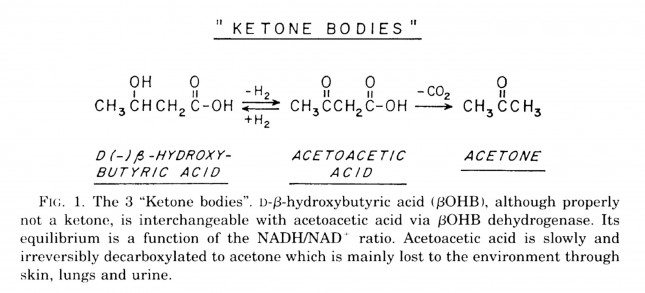
Acetoacetic acid and B-OHB live in reversible equilibrium (on the left), but once acetoacetate is converted to acetone (on the right) there’s no going back.
Now take a look at the figure below, from this 2001 paper. This is another rendition of the figure above showing the Krebs Cycle, but here you can see where B-OHB and acetoacetate enter the picture.
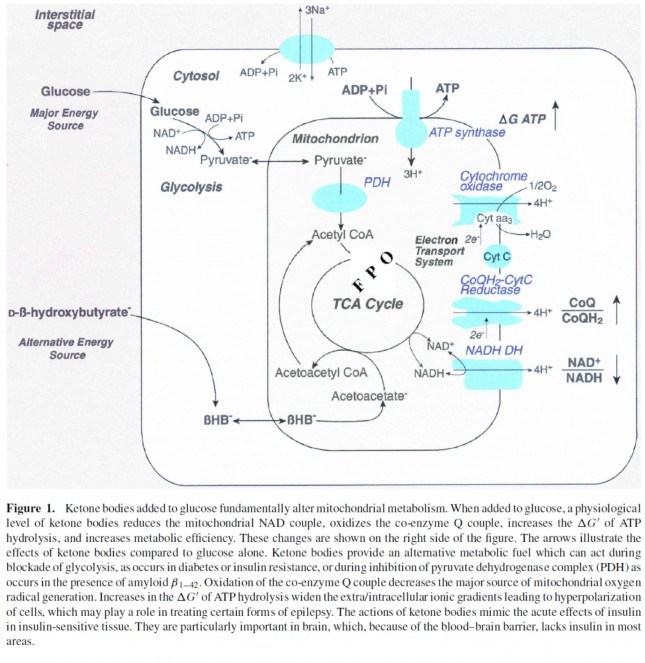
The reason a starving person can live for 40-60 days is precisely because we can turn fat into ketones and convert ketones into substrate for the Krebs Cycle in the mitochondria of our neurons. In fact, the more fat you have on your body, the longer you can survive. As an example of this, you may want to read this remarkable case report of a 382 day medically supervised fast (with only water and electrolytes)! If we had to rely on glucose, we’d die in a few days. If we could only rely on protein, we’d live a few more days but become completely debilitated with muscle wasting.
The graph below, also from the Cahill and Veech paper, shows the blood chemistry of a person starving for 40 days. Within about 3 days, a starving person’s level of glucose stops falling. Within about 10 days they reach a steady-state equilibrium with B-OHB levels exceeding glucose levels and offsetting most of the brain’s need for glucose. In fact, the late George Cahill did an experiment many years ago (probably would never get IRB approval to do such an experiment today) to demonstrate how ketones can offset glucose in the brain. Subjects with very high levels of B-OHB (about 5-7 mM) were injected with insulin until glucose levels reached 1 mM (about 19 mg/dL)! A normal person would fall into a coma at glucose levels below about 40 mg/dL and die by the time blood glucose reached 1 mM. These subjects were completely asymptomatic and 100% neurologically functional.
The last point I’ll make on the starving patient is that, as you can see in the figure below, the glucose level normalizes at about 65-70 mg/dL (about 3.7 mM) within days of fasting, despite no sources of exogenous glucose. Why? Because with so much fat being converted into B-OHB and acetoacetic acid by the liver, a significant amount of glycerol (the 3-carbon backbone of triglycerides) is liberated and converted by the liver into glycogen. As an aside, this is why someone in nutritional ketosis – even if eating zero carbohydrates – still has about 50-70% of a normal glycogen level, as demonstrated by muscle biopsies in such subjects.
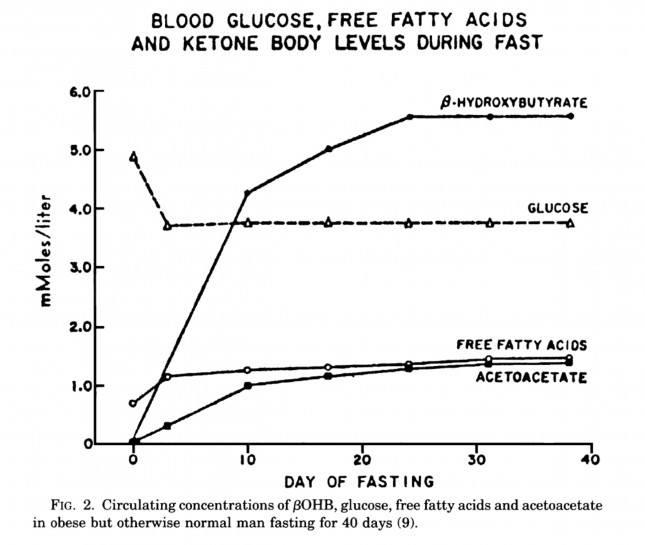
Take home message #3: We evolved to produce ketone bodies so we could not only tolerate but also thrive in the absence of glucose for prolonged periods of time. No ability to produce ketone bodies = no human species.
Last point of background: Everything I’ve just presented is based on data from starving subjects. If one restricts carbohydrate intake, typically to less than about 20-50 gm/day (dependent on timing and carbohydrate composition), and maintains modest but not high protein intake (because protein is gluconeogenic – i.e., protein in excess will be converted to glycogen by the liver), one can induce a state referred to as “nutritional ketosis” with similar physiology to what I’ve just presented without resorting to starvation. Why you’d do this is something I will discuss later.
One other housekeeping issue: Ketosis versus DKA?
In a separate post, I explained the difference between nutritional ketosis (NK) and diabetic ketoacidosis (DKA). If this distinction is not clear, I’d suggest giving this separate post a quick skim for a refresher. DKA is a pathologic (i.e., harmful) state that results from the complete or near absence of insulin. This occurs in the setting of type 1 diabetes or very end-stage type 2 diabetes, and often as the result of a physiologic insult (e.g., an infection) where the patient is not receiving sufficient insulin to bring glucose into his cells. A person with a normal pancreas, regardless of how long he fasts (including the fellow I reference above who fasted for 382 days!) or how much he restricts carbohydrates, can not enter DKA because even a trace amount of insulin will keep B-OHB levels below about 7 or 8 mM, well below the threshold to develop the pathologic acid-base abnormalities associated with DKA. Let me reiterate, it is physiologically impossible to induce DKA in anyone that does not have T1D or very, very, very late-stage T2D with pancreatic “burnout.”
Embarrassing admission: I remember exactly where I was sitting in a clinic at Johns Hopkins in 2002 explaining to (admonishing, really) a patient who was on the Atkins diet how harmful it was because of DKA. I am so embarrassed by my complete stupidity and utter failure to pick up a single scientific article to fact check this dogma I was spewing to this poor patient. If you’re reading this, sir, please forgive me. You deserved a smarter doctor.
In Part II of this post I’ll tackle the questions I know folks still have on their mind (below). Until then, re-read this post to make sure you really understand this physiology. You’re already 10 steps ahead of the next person.
- Is there a “metabolic advantage” to being in ketosis?
- Are there dangers of being in ketosis?
- What are the most important things you need to know about getting into (or staying in) ketosis?






Absolutely the best description of the many I’ve read on ketosis. We non-scientist/medicos really appreciate such a clear rendering of the information. All I know is that I feel great on ketones after the first couple of headachy days (a couple aspirin usually does the trick). Thank you.
PS- Everyone who teaches has at some point realizes to their shame that they taught in error, but learning comes with trial and error, even for the teacher. At least you saw the error, and sadly too many won’t even try.
I have a question I have not found an answer to. There has always been this notion that lowering calories past a certain point will decrease metabolism. I see the logic in this ….. if you are not in nutritional ketosis. If when in NK you are burning reserve fuel in the form of body fat and your body does not know the difference between fat fuel that comes from the diet and fat fuel that comes from your fat stores….. wouldn’t it be impossible to decrease your metabolism if you decrease you calorie intake to say 800/day. As long as you were holding protein intake to what it should be….. and holding all other variables constant shouldn’t your metabolism also hold constant.
Very difficult to say…one could argue that as your weight drops, so too does energy expenditure, as you’re moving less weight around. Look at the work of Rudy Leibel and Michael Rosenbaum for this.
Hi Dr.Attia! I’ve been in ketosis for a little over 3 months now, I plan on trying to make this a long term way of life, the benefits are too great to go back to dealing with the glucose/insulin rollercoaster. My question is this: I’ve lost about 25 pounds and i’m trying to stabilize my weight because i’m getting pretty thin and would like to add muscle. I know you hit the gym and are ketotic so I was wondering what you do to add/keep muscle and what you would suggest. I’m going to start using creatine to add some hydration in the muscles so that would be a little bit of a starting point for me…Please let me know, I appreciate all the info!-Ken
Check some of the other comments in response to similar questions. Protein type, protein amount, and protein timing all play a role. Of course, training plays a role, also, so “being in the gym” isn’t really enough…it’s probably more about how one trains, in addition to the other points.
I don’t think ketosis is the optimal state for hypertrophy, but it’s certainly possible.
Hi Dr. Attia! I’ve really enjoyed your website & blog — thanks for dedicating so much energy to sharing this info. My husband is Type 1 Diabetic (diagnosed at 30, he is now 49). After I saw a collective report of 5 years of A1Cs, in which NONE were below 8 (the most recent was 9.2) I knew we had to make a drastic change. He is very fit / athletic and shows no (apparent) complications…yet. But continuing at those levels, there will be no avoiding them. His endocrinologist wants him on a pump… but that’s the extent of his guidance. After much research last month, I literally stumbled upon the primal/paleo diet, and later on your ketogenic “experiment” as well as work done by Dr. Richard K. Bernstein (T1D endocrinologist). Three weeks ago, both my husband and I (in support of his efforts) began eating a strict paleo diet, but I am struck by how little information is available for Type 1 diabetics on low-carb diets. I absolutely do NOT want to do more harm than good… and I thoroughly understand the difference between ketogenic & ketoacidosis — as long as he’s testing & taking insulin he requires, he’s safe. My concern is over clotting changes in his blood, or any other factors which may be affected by our new way of eating. We are both committed to making this a permanent change, and (other than occasional light-headedness) we both feel better. Do you have any trusted resources you can share, for the management of T1D using a ketogenic diet? Thanks in advance for your reply — we have scheduled him with a different endocrinologist to help track his info… hopefully she does not push the American Diabetic Association’s high-carb “just take more insulin” diet.
Heidi, certainly being a T1D poses a few extra challenges, but I know a number of folks with T1D who have absolutely changed their life with this dietary approach, all based on the approach of Dr. Bernstein. Someone with T1D will always require insulin, of course, but it’s not uncommon to reduce requirement by 80% over just a few months.
A friend of mine is the most obsessive T1D I know and I’ve been begging him to start a blog specifically for T1D patients…hopefully he will soon. He’s so completely knowledgeable about the ins and outs. For many years he would fly from Florida (where he lives) to NY to see Dr. B.
Sure there is: Bernstein’s Diabetes Solution
Peter, was it only last Wednesday that I read about your TEDMED talk and decided to read your articles? Seems like much longer. I am a 50 yo female physician (psychiatry internal medicine actually) and have been working with patients to help them reach their weight loss goals. I’ve been frustrated (and so have they) at what they feel are conflicting messages on what to eat and how to lose weight. Many have failed so many times that I’m amazed that they keep coming back to my support group.
About 15 years ago I took off an extra 35lbs and am at a good weight and have maintained it without too much trouble. My diet consists of nearly pure carbs, as much sugar as I can eat. Every time I eat them I think to myself, “Wow, you are setting a bad example.” Still, I continued to do it. I couldn’t even give up Chocolate for Lent!
Something in what I read on Wednesday really clicked with me. I’m not sure what it was, probably the mention of all that I, as a physician, already know….with a new twist in my mind about the true gravity of what I was subjecting my body to every day…specifically high insulin levels.
In any case, starting Wednesday I decided that I wasn’t going to eat sugar anymore, and was going to try to go VERY low carb….and somehow I have managed to do it! I thought that I craved sugar and carb and couldn’t survive without them. I even joked about how I could never have been a pioneer because there wasn’t any way to transport the amount of Ice Cream that I needed. Since I changed my mind on Wednesday, when I even think of eating carbs (which actually isn’t very often), I think something along the lines of, “what other poison do you want to add to that.” and whatever excuse I was giving to myself just melts away and I don’t do it.
Up to one week ago I was snacking ALL the time, ALL the time. Starting Wednesday I told myself to Not do that. I eat 3 meals a day, I log my calories in an online program and it looks like I’ve been eating about 65carbs/day. I’ve been HUNGRY a couple of times, but have NOT been craving sugar. I’ve walked past the candy jar 100 times….and when typically I would have grabbed a piece of candy every time, I haven’t had ONE, NOT ONE since Wednesday.
I’m not sure where this is leading, but I think that I can do this for the rest of my life…and I won’t have to keep berating myself for eating foods that I know have no nutritional value and actually harm me. Thanks for thinking about this. I will try to keep up with your research, seems like you’ve got an open mind and if you find a better idea you will let us know.
Thanks for sharing.
Hi Peter,
I live in the UK and I recently read Gary Taubes book on”why we get Fat”. I know the book mentioned Ketosis briefly, anyway great book!
I have previously been on a Ketogenic diet Which involved only have meal replacement four times per day, which equaled 500 calories per day and I did this diet for >12 weeks straight. Excellent diet lost >4 stones in weight! Experienced the high energy, no afternoon blues, my sleep apnea went and of course loved my new wardrobe!
Now I’m at a place of having regained all the weight and an extra stone, tiredness has returned etc.
This time round I want to be in control! I know Ketogenic diet works! But just need to adapt it to real food!
Please can you show me, literally? I get the science (to a point) but translating into know practically what to buy in a supermarket , with all these labels with “hidden sugar” or substances to which our bodies would respond to it as sugar/starch, is so confusing.
Please help 😉
Do you mean fly to the UK and move in with you and “literally” show you? 🙂 I think I’m doing all I can, Patricia. Looks like you’ve already figured it out.
I don’t know if I should be picky with the chemistry but it is “acetoacetate” and not “acetoacetone” in the technical part on top.
acetoacetone would be the same thing with a CH3 group instead of the OH group on the right
anyway it was very interesting to read
Hi Peter,
Lol! Literally in terms of what do I need to consider, in terms of calories per day. The number of calories you consume, you’ve said related to your lifestyle or the level if training you did. I work in an office and I do minimal exercise. The diet I did previously was 500 calories per day. In an earlier post someone mentioned basal metabolic rate. Does this need to be considered in order for me to consume the right ratios. This is the part that’s a little confusing.
How do I translate all this information to literal day to day planning of meals (what should be included) to achieve ketosis with real food.
Thank u 🙂
Patricia, I’ll give you my 2 cents. I wouldn’t worry too much about caloric intake especially at first. The primary thing you need to do the first few weeks is to start eating a healthy low carb diet. Don’t worry about portion size when you’re adapting. You will naturally start eating less over time as you get use to the new way of eating. Don’t just zone in on a few different recipes, give yourself a variety of things to eat. There are a lot of good resources on the web for healthy low carb eating. See this blog and Mark Daily Apple for starters. You will not lose weight as fast as on the 500 cal per day diet, but the weight will come off and if you continue to follow the plan you will have a lifestyle you can maintain. Good luck. Dave
Thanks Dave 😉
Don’t feel too bad about the DKA incident at Johns Hopkins, Peter. Not all of us listen to our doctors anyway! I was in the hospital a few years ago with pneumonia, refused the Prednisone, refused the Heparin, refused something else I don’t remember what, reluctantly accepted the antibiotics, refused the food (mystery mashed potatoes and chocolate cake) they made me sign legal papers, they had someone come “talk sense” to me – and then the doctor – exasperated and exhausted herself – told me that I was being unreasonable in thinking that I needed SLEEP (nurses repeatedly waking me up for their constant checks.) I even made my husband bring a jar of coconut oil from home. Needless to say I wasn’t one of the “good” patients. 🙂
Thanks for the great TED talk, which led me to here, because I didn’t know about you. I am very happy to see that you have teamed up with Gary Taubes, who wrote my favorite book of all time, GCBC. (A couple of others, Dr. Eades and Dr. Lustig – glad to see him getting some play in the media too.)
I did the same thing that you did: I experimented on myself. I’ve been phasing back and forth between low-carb and zero-carb, depending on the season, for several years now, and have found myself naturally falling into cycles of IF too. Most days I only want to eat one main meal, some days I eat more, some seasons I eat a few more veggies (summer.) I lost a bunch of weight (50 lbs), cured my husband’s gout (he isn’t happy about giving up his dried fruit snacks, but the memory of severe pain serves as an excellent incentive) and my Lyme disease is 95% in remission with no antibiotics – (if I eat even a little bit of sugar however, the twinges of pain manifest within a couple of hours.) We have no other health problems, take no prescriptions at all, we feel great, and our brains are clear.
But despite our results, our friends and family all think we are crazy to eat the way we do, so I am very glad to see yet another doctor who “gets it.” Bless you and Gary T. both.
Thanks very much, Sarah. We may be crazy, actually.
thank you Peter for explaining nutritional ketosis. I am on a ketogenic diet myself since app. 10 months (with HCG which also leads to nutritional ketosis and VLCD for maintance) having lost 75 Pounds so far.
Dr. Jan Kwasniewsky, a polish doctor has a very good book on ketogenic diet, promoting a high fat, moderate protein and very low carbohydrate diet (Title: the optimal diet). He also claims high success with T1D.
Simply written for any layman to understand with recepes (typical polish so not to everybodies taste, but can easily be altered).
I am also a T2D, but off all medication since I started the ketogenic diet.
I have one question: I was told that being in lypolysis and being ketogenic is not the same. To me it makes no sense but maybe I am missing an important point.
Correct. Future blog post will explain this.
“In fact, the late George Cahill did an experiment many years ago (probably would never get IRB approval to do such an experiment today) to demonstrate how ketones can offset glucose in the brain. Subjects with very high levels of B-OHB (about 5-7 mM) were injected with insulin until glucose levels reached 1 mM (about 19 mg/dL)! A normal person would fall into a coma at glucose levels below about 40 mg/dL and die by the time blood glucose reached 1 mM. These subjects were completely asymptomatic and 100% neurologically functional.”
+
Cancer eats sugar.
=
Cure for cancer?
Will address in part III of this series.
I just found this study and it distressed me. I have been doing this low card diet <80 for 2 weeks. Its been a struggle. Never realized what a sweet tooth I had. Anyway, I'm doing fine, not thriving yet, but persistent, and I found this article. I attached a description. Anyway I do worry about bone density but mostly I worry about acid environment. I have been testing pH and it runs low…
https://www.familypracticenews.com/news/child-adolescent-medicine/single-article/bone-growth-affected-by-ketogenic-diet-in-children-with-epilepsy/841b08dd834bb1dc8c081231f9af7f7c.html
VITALS
Major finding: Participants on the ketogenic diet demonstrated a mean bone mineral density lumbar Z-score decrease of 0.1756 units/year. Bone loss was greater in children who had higher baseline Z-scores (–0.28 vs. –0.04 units/year).
Data source: A prospective, longitudinal study of 29 children who were treated with the ketogenic diet for more than 6 months during 2002-2009.
Disclosures: The study was partially funded by Pfizer Australia. Dr. Mackay did not have any financial disclosures.
The findings highlight the risks of a ketogenic diet, which relies on fat metabolism to induce ketoacidosis. A neutral pH is necessary to mobilize calcium from bone, Dr. Mackay said.
Ellen, I’m reasonably familiar with this literature, though a few explanations may exist:
1. (relative?) Protein deficiency, which is pretty common on these diets for this population;
2. Suitability of these diets in children which rapid bone growth (vs. adults);
3. I did not read the study, but if not a randomization, not sure “matched controls” didn’t have other differences in their lives to cause the difference in bone density (e.g., activity).
Thank you so much for this blog and all of the amazing information.
I have two questions related to nutritional ketosis:
Thomas Seyfried and others suggest that to reap the metabolic benefits of ketosis (particularly for the brain to be able to utilize B-OHB), that one must be in a state of “therapeutic” ketosis where the blood ketones are higher than blood glucose. He suggests a 3 day fast to get the glucose low enough to achieve that “therapeutic” state. My question is, my blood glucose is usually in the low 80’s and my B-OHB is most always between 2-4 (Except maybe first thing in the morning). Does this mean I am not reaping the metabolic benefits of ketosis, especially my brain? Should one really aim to be in this optimal therapeutic range where glucose ketones are higher than glucose? I thought I was doing quite well, but now I’m not sure what the goal really is. Is it just having ketones in the 2 or above range or do we want Blood Glucose to get as low as 55-65 mg?
My second question is that Seyfried, Dominique D’Agostino, etc, suggest that a ketogenic diet is only EFFECTIVE if calories are restricted to between 1,000-1,500 calories/day. They say that eating an “unrestricted” caloric ketogenic diet can be quite harmful. I would love some clarification about this. IF I ate that few calories, my weight would drop (and it did) beyond what I believe is optimal. I am working hard to eat over 1600 calories/day to get back up to a weight of 114 lbs for my 5’3 height. But then this results in what they refer to as an “unrestricted ketogenic diet”, which they suggest, like I said, doesn’t get you any of the metabolic benefits of ketosis. Please help to clarify. I am so confused!!
To question 1, I’m not sure about that, but I’d love to see the data. Without starvation or extreme caloric restriction, it’s difficult to get BHB (in mM) higher than glucose (in mM). Not impossible, but at least for me, it only happens after a very long bike ride at about 60-65% VO2 max. So while I agree that a threshold is probably necessary, which is likely much higher than the typical 0.5 to 1.0 mM most people think of, I’m not sure this rule applies. Furthermore, in most folks BHB and AcAc exist in a 1:1 ratio, so while we measure BHB in plasma, we ought to double it (roughly) to calculate total ketones. Under this assumption, I’d have an easier time believing this.
As to your second question I can’t really speak for them, but I know Dominic (not Dominique) well. In fact, I’ll be spending 2 days with him next week. So I guess I can ask him.
Hi Peter,
With regards to question 1, I’m glad to hear this isn’t your experience either! What do you mean by a 1:1 ratio with BHB and AcAC? Isn’t the later something yo measure on a urine stick? How would you get a number for that?
I’d love to hear what Dom says about keeping calories between 1,000-1,500 to get the benefits of ketosis. I’m wondering if he’s referring to patent’s with cancer – in which case the answer to question 1 might be the same. Please let me know how he responds.
One other question: A practitioner I work with recommends protein pulsing (avoiding protein 2-3/days a week). This is supposed to have huge benefits for the immune system, etc. I have started doing that at least twice/wk. by simply eating more fat and getting my protein down to about 4-5% of my intake. I’m curious if you know anything about this way of restricting protein. Interestingly, I’ve observed that on the mornings following a non protein day, my ketones are significantly more elevated. Would love your thoughts.
Lastly, do you have a calculation for figuring out the amount of protein you eat per day. I’ve read that one only needs around .8-1 gram per kg of lean body mass. For me, who recently had my body mass tested, this would only amount to 39 grams of protein/day. I”ve also read that one should get about .6-1kg per LB (instead of kg) of lean body mass. If I went with this calculation, then I’d be getting more like 55 grams of protein/day. I find this issue very confusing. I’d love your thoughts about calculating optimal protein amounts.
Thanks so much. I really appreciate being able to have this dialogue.
Best,
Robin
Yes, AcAc is more typically measured in urine, but this is qualitatively. It can be measured quantitatively in blood, but is typically only done for research purposes.
HI Peter,
I’d love your thoughts about the second part of the question of skipping protein altogether 2-3 days /week (Ron Migurney has a book about this called Protein cycling). Apparently this activates the immune system and good stuff happens, including increased autophagy, etc. .
I’m wondering if you have any thoughts about the possible negative effects of skipping protein 2-3/days/ week – and whether you think it is important tn to eat protein every day in order to maintain muscle mass. I am 5’3 and weigh about 114. I also have osteoporosis, so am very concerned about maintaining and gaining lean muscle mass. My body fat percentage is 22.9 and my lean muscle mass is 87 lbs.
I’d love your thoughts about the protein skipping as well as what you’d suggest in terms of protein amount. I’ve been eating about 55 grams of protein/day when I do eat protein (12% of calories). Not sure if this is sufficient or not. I do high intensity strength training 2x/week and interval cardio 1x/week, Am I getting too little, too much, etc, for my goal of maintaining my weight and muscle mass?
Much appreciative of your help with sorting this out!!
I have no insight to this, but can’t imagine a great rationale for skipping protein, especially if physically active.
Since being on low carb, high fat diet, I have had leg cramps. I take supplements of Ca, Mg, K, Vit. D regularly. Then I read about sodium deficiency caused by this diet so started drinking 1 tspn Bovril in a mug of hot water twice a day. This worked dramatically for a few weeks but then the cramps came back at night but not nearly as severely. As I have osteopaenia, I am concerned about taking too much salt as it can cause Ca loss from the bones. What should I do?
The fact that the muscle cramps were gone temporarily seems to indicate that you removed the cause of your cramps. Given that this happened once you increased sodium, the sodium or the sodium in conjunction with at least one of the other supplments seems to be the responsible for the reduction in cramps.
Because there are several causes for muscle cramps, what you can do is to test for yourself what brings you relief. Try increasing/reducing one supplement at a time to figure out which one helps. I would do it for several weeks, so you can see if it really helps.
There are four things I noticed (which are missing from your post):
1. You say you take your supplements regularly. What does that mean exactly? Regularly could mean “every 2 hours” and it could mean “every year on 15th Feb”.
2. What dosages are you taking?
3. Which form are you taking?
4. When are you taking them? (Meaning: approx. which time of the day?)
The reason I am asking these question is that I noticed for myself that they matter. Several years ago I had leg cramps several times a month, although this happened primarily during winter. There are several things I noticed during this time:
1. Mg really helps. Nowadays, I take about 400mg of Mg about 1h before bedtime; sometimes I additionally take 100mg-200mg during the day, which I put in my drinking water; to which I also add salt (about 2g-4g/day, depending on what I eat, my exercise level, the temperature etc.). Mg was – for me – the most important supplement. I chose MgCl as a form, because I could use it topically or in a bath, it definitely works for me and it’s inexpensive (depending on brand, of course)
2. Cold feet/legs. One of the most most important triggers I noticed for myself are cold feet/legs. You might wanna make sure your feet or at least legs stay warm during the night. Long pants and maybe even socks can help a lot.
3. Sodium does help me. I added the 2g-4g of salt/day when I got into ketosis. I seem to do better by not overdoing salt, but especially when I am sweating a lot due to exercise and/or weather, 2g don’t seem to be enough. Given that you are taking potassium, you might also want to experiment with reducing K instead of increasing sodium to see how the sodium/potassium ratio does affect you.
I don’t know anything about osteopaenia, but I don’t think it would heart to try a little more salt/Bovril for some time to see if it helps. I would be surprised if a few grams of salt a day would cause or be detrimental for osteopaenia in someone who eats healthy and takes D3 and K2 as a supplement. Maybe high intensity exercise would also help in increasing bone density, although osteopaenia in itself does not seem to be a problem as long as it doesn’t progress to osteoporosis.
Those are just a few thoughts. Maybe some of it can help. It seems to me that you are on the right track. And testing things for yourself really is the best thing you can do; especially when it comes to things like muscle cramps which seem to be caused by a lot of different factors.
Hi Peter,
I am wondering if you are aware of any ideas of how to measure the state of keto-adaptation. I find the current approach of “get your blood ketone levels >=0.5mmol/l and wait 2-4 weeks in the hopes of being keto-adapted” a bit lacking and am wondering if you are aware of any work being done to improve this situation.
I also think that the argument against urine ketone sticks is also true for blood ketone sticks to a lesser degree, in that blood BOHB levels only tell you how much is there, but neither how much is produced nor (which is probably the most important information) how much is actually used. So measuring blood ketone levels, while a step up from ketostix, seems to be only a stopgap and we will probably need something better for accurately assessing the state of ketosis in the future. Given that nutritional ketosis seems to catch the interest of many people, hopefully more work will be done in this field.
I am not. The “real” way would probably involve some combination of muscle biopsy and complex serum assays. I’m not sure the numbers — levels or duration — are perfect. A more conservative estimate based on my experience is: morning fasting levels of BHB between 1 and 2 mM repeatedly and about 3-6 months.
Peter, if you have had your pancreas surgically removed can you live without insulin if you follow a ketogenic diet? If so, can a Type 1 diabetic also survive without insulin on ketogenic diet?
No, one needs insulin to live. I’ve seen a few anecdotal cases of people with T1D on KD being free of insulin, but it suggests they have a tiny bit of beta cell function remaining.
Peter.
What do you think of ApoE tests and the implications from them? Specifically, do you think that genotype might explain our individual reactions to diets, meds, alcohol, etc.? (Many sites and researchers are suggesting that we should take ApoE genotype into account for all those things, but I’m sleptical until I see more research.)
My test came back as ApoE 2/4, which some say is the same as being a 3. Since only 2% of the population is like me (2/4), I doubt anyone will do a definitive study to confirm that anytime soon. From my n=1 experience, the no-carb, high-fat, moderate exercise approach is working fine. I was on a clear path toward metabolic syndrome before I found this blog. I’ve had great success since, and I’m not inclined to change because someone says ApoE 3’s (and 2/4’s) should eat more carbs and less fat.
Would you mind telling us what you think of these tests, and what type you are?
Thanks!
Richard
It’s one of the few genes I pay attention to since it gives, I believe, actionable information. The 2/4 allele combo is an interesting one. As you note, it’s about 2% of the population (by contrast 2/2 is 1% of the population, about the same as 4/4), so we’re not sitting on piles of data. I am mostly use apoE for 3/4 and 4/4 alleles. I’m a 3/3.