One night, as I alluded to in this post, Tim and I were having dinner and the topic of cancer came up. Personally and professionally I have a great interest in cancer, so when Tim asked if I could write something about cancer that was: (i) interesting to a broad audience, (ii) not technically over the top, (iii) not my typical 5,000 word dissertation, (iv) yet nuanced enough for his readers, I agreed to give it a shot, in about 1,000 words. (The content of this blog went up on Tim’s blog last week, but I’ve reproduced it here, less Tim’s commentary.)
Semantics and basics
Before jumping into this topic I want to be sure all readers — regardless of background — have a pretty good understanding of the ‘basics’ about cancer and metabolism. In an effort to do this efficiently, I’ll list concepts here, such that folks can skip them if they want to, or refer back as necessary. This way, I don’t need to disrupt the ‘story’ with constant definitions. (Yes, I realize this is sort of cheating on my 1,000 word promise.)
Cancer – a collection of cells in our bodies that grow at roughly normal speeds, but that do not respond appropriately to cell signaling. In other words, while a collection of ‘normal’ cells will grow and stop growing in response to appropriate messages from hormones and signals, cancer cells have lost this property. Contrary to popular misconception, cancers cells do not grow especially fast relative to non-cancer cells. The problem is they don’t ‘know’ when to stop growing.
Metabolism – the process of converting the stored energy in food (chemical energy contained mostly within the bonds of carbon and hydrogen atoms) into usable energy for the body to carry out essential and non-essential work (e.g., ion transport, muscle contraction).
ATP – adenosine triphosphate, the ‘currency’ of energy used by the body. As its name suggests, this molecule has three (tri) phosphates. Energy is liberated for use when the body converts ATP to ADP (adenosine diphosphate), by cutting off one of the phosphate ions in exchange for energy.
Glucose – a very simple sugar which many carbohydrates ultimately get broken down into via digestion; glucose is a ring of 6-carbon molecules and has the potential to deliver a lot, or a little, ATP, depending on how it is metabolized.
Fatty acid – the breakdown product of fats (either those stored in the body or those ingested directly) which can be of various lengths (number of joined carbon atoms) and structures (doubled bonds between the carbon atoms or single bonds).
Aerobic metabolism – the process of extracting ATP from glucose or fatty acids when the demand for ATP is not too great, which permits the process to take place with sufficient oxygen in the cell. This process is highly efficient and generates a lot of ATP (about 36 units, for example, from one molecule of glucose) and easy to manage waste products (oxygen and carbon dioxide).
The process of turning glucose and fatty acid into lots of ATP using oxygen is called ‘oxidative phosphorylation.’
Anaerobic metabolism – the process of extracting ATP from glucose (but not fatty acids) when the demand for ATP is so great that the body cannot deliver oxygen to cells quickly enough to accommodate the more efficient aerobic pathway. The good news is that we can do this (otherwise a brief sprint, or very difficult exertion would be impossible). The bad news is this process generates much less ATP per carbon molecule (about 4 units of ATP per molecule of glucose), and it generates lactate, which is accompanied by hydrogen ions. (Contrary to popular belief, it’s the latter that causes the burning in your muscles when you ask your body to do something very demanding, not the former).
Mitochondria – the part of the cell where aerobic metabolism takes place. Think of a cell as a town and the mitochondria as the factory that converts the stored energy into usable energy. If food is natural gas, and usable energy is electricity, the mitochondria are the power plants. But remember, mitochondria can only work when they have enough oxygen to process glucose or fatty acids. If they don’t, the folks outside of the factory have to make due with suboptimally broken down glucose and suboptimal byproducts.
DNA – deoxyribonucleic acid, to be exact, is the so-called “building block” of life. DNA is a collection of 4 subunits (called nucleotides) that, when strung together, create a code. Think of nucleotides like letters of the alphabet. The letters can be rearranged to form words, and words can be strung together to make sentences.
Gene – if nucleotides are the letters of the alphabet, and DNA is the words and sentences, genes are the books – a collection of words strung together to tell a story. Genes tell our body what to build and how to build it, among other things. In recent years, scientists have come to identify all human genes, though we still have very little idea what most genes ‘code’ for. It’s sort of like saying we’ve read all of War and Peace, but we don’t yet understand most of it.
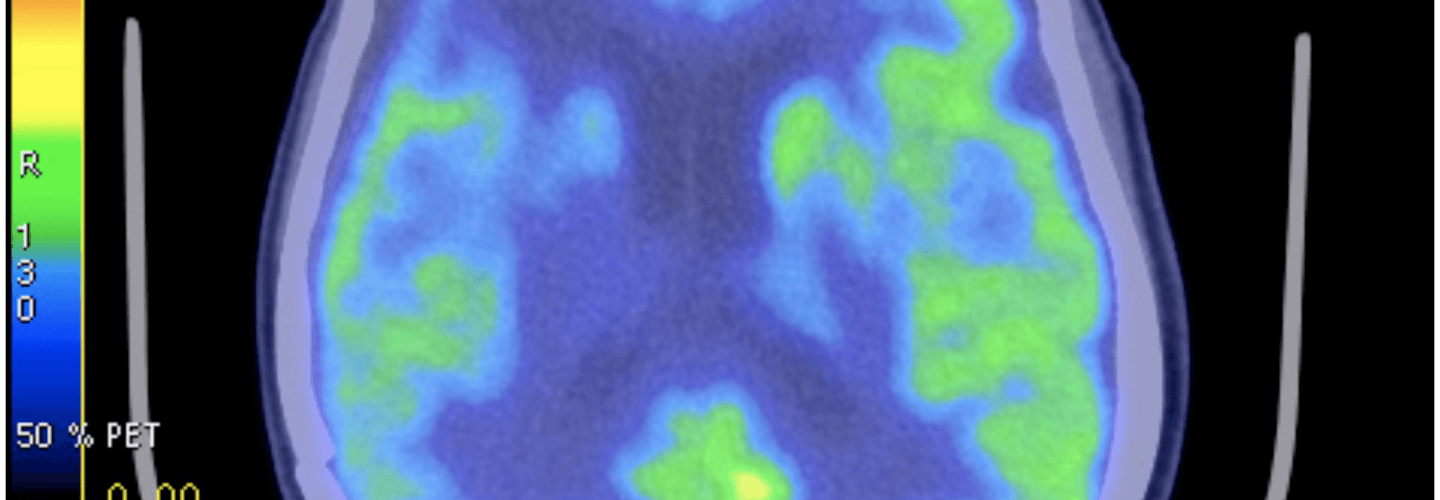
FDG-PET – a type of ‘functional’ radiographic study, often called a ‘pet scan’ for short, used to detect cancer in patients with a suspected tumor burden (this test can’t effectively detect small amounts of cancer and only works for ‘established’ cancers). F18 is substituted for -OH on glucose molecules, making something called 2-fluoro-2-deoxy-D-glucose (FDG), an analog of glucose. This molecule is detectable by PET scanners (because of the F18) and shows which parts of the body are most preferentially using glucose.
Phosphoinositide 3-kinase – commonly called PI3K (pronounced “pee-eye-three-kay”), is an enzyme (technically, a family of enzymes) involved in cell growth and proliferation. Not surprisingly, these enzymes play an important role in cancer growth and survival, and cancer cells often have mutations in the gene encoding PI3K, which render PI3K even more active. PI3Ks are very important in insulin signaling, which may in part explain their role in cancer growth, as you’ll see below.
The story (in about 1,000 words, as promised)
In 1924 a scientist named Otto Warburg happened upon a counterintuitive finding. Cancer cells, even in the presence of sufficient oxygen, underwent a type of metabolism cells reserved for rapid energy demand – anaerobic metabolism. In fact, even when cancer cells were given additional oxygen, they still almost uniformly defaulted into using only glucose to make ATP via the anaerobic pathway. This is counterintuitive because this way of making ATP is typically a last resort for cells, not a default, due to the very poor yield of ATP.
This observation begs a logical question? Do cancer cells do this because it’s all they can do? Or do they deliberately ‘choose’ to do this? I’m not sure the answer is entirely clear or even required to answer the macro question I’ve posed in this post. However, being curious people we like answers, right?
The first place to look is at the mitochondria of the cancer cells. Though not uniformly the case, most cancers do indeed appear to have defects in their mitochondria that prevent them from carrying out oxidative phosphorylation.
Explanation 1
Cancer cells, like any cells undergoing constant proliferation (recall: cancer cells don’t stop proliferating when told to do so), may be optimizing for something other than energy generation. They may be optimizing for abundant access to cellular building blocks necessary to support near-endless growth. In this scenario, a cancer would prefer to rapidly shuttle glucose through itself. In the process, it generates the energy it needs, but more importantly, it gains access to lots of carbon, hydrogen, and oxygen atoms (from the breakdown of glucose). The atoms serve as the necessary input to the rate-limiting step of their survival — growth. The selection of cancer cells is based on this ability to preferentially grow by accessing as much cellular substrate as possible.
Explanation 2
Cells become cancerous because they undergo some form of genetic insult. This insult – damage to their DNA – has been shown to result in the turning off of some genes (those that suppress tumor growth) and/or the activation of other genes (those that promote cell growth unresponsive to normal cell-signaling). Among other things, this damage to their DNA also damages their mitochondria, rendering cancer cells unable to carry out oxidative phosphorylation. So, to survive they must undergo anaerobic metabolism to make ATP.
Whichever of these is more accurate, the end result appears the same – cancer cells almost exclusively utilize glucose to make ATP without the use of their mitochondria. A detailed discussion of which explanation is better is beyond the scope of my word allotment, and it’s not really the point I want to make. The point is, cancer cells have a metabolic quirk. Regardless of how much oxygen and fatty acid they have access to, they preferentially use glucose to make ATP, and they do it without their mitochondria and oxygen.
So, can this be exploited to treat or even prevent cancer?
One way this quirk has been exploited for many years is in medical imaging. FDG-PET scans are a useful tool for non-invasively detecting cancer in people. By exploiting the obligate glucose consumption of cancer cells, the FDG-PET scan is a powerful way to locate cancer (see figure).
You can probably tell where I’m leading you. What happens if we reduce the amount of glucose in the body? Could such an intervention ‘starve’ cancer cells? An insight into this came relatively recently from an unlikely place – the study of patients with type 2 diabetes.
In the past few years, three retrospective studies of patients taking a drug called metformin have shown that diabetic patients who take metformin, even when adjusted for other factors such as body weight and other medications, appear to get less cancer. And when they do get cancer, they appear to survive longer. Why? The answer may lie in what metformin does. Metformin does many things, to be clear, but chief among them is activating an enzyme called AMP kinase, which is important in suppressing the production of glucose in the liver (the liver manufactures glucose from protein and glycerol and releases it to the rest of the body). This drug is used in patients with diabetes to reduce glucose levels and thereby reduce insulin requirement.
So, the patients taking metformin may have better cancer outcomes because their glucose levels were lower, or because such patients needed less insulin. Insulin and insulin-like growth factor (IGF-1) also appear to play an integral role in cancer growth as recently demonstrated by the observation that people with defective IGF-1 receptors appear immune to cancer. Or, it may be that activation of AMP kinase in cancer cells harms them in some other way. We don’t actually know why, but we do know that where there is smoke there is often fire. And the ‘smoke’ in this case is that a relatively innocuous drug that alters glucose levels in the body appears to interfere with cancer.
This may also explain why most animal models show that caloric restriction improves cancer outcomes. Though historically, this observation has been interpreted through the lens of less ‘food’ for cancer. A more likely explanation is that caloric restriction is often synonymous with glucose reduction, and it may be the glucose restriction per se that is keeping the cancer at bay.
Fortunately this paradigm shift in oncology – exploiting the metabolic abnormality of cancer cells – is gaining traction, and doing so with many leaders in the field.
Over a dozen clinical trials are underway right now investigating this strategy in the cancers that appear most sensitive to this metabolic effect – breast, endometrial, cervical, prostate, pancreatic, colon, and others. Some of these trials are simply trying to reproduce the metformin effect in a prospective, blinded fashion. Other trials are looking at sophisticated ways to target cancer by exploiting this metabolic abnormality, such as targeting PI3K directly.
To date, no studies in humans are evaluating the therapeutic efficacy of glucose and/or insulin reduction via diet, though I suspect that will change in the coming year or two, pending outcomes of the metformin trials.
Last point (beyond my 1,000 word allotment)
Check out this blast from the past! Gary Taubes, who is currently working hard on his next book, came across the article the other day from 1887.
Influences
I’ve been absurdly blessed to study this topic at the feet of legends, and to be crystal clear, not one thought represented here is original work emanating from my brain. I’m simply trying to reconstruct the story and make it more accessible to a broader audience. Though I trained in oncology, my research at NIH/NCI focused on the role of the immune system in combating cancer. My education in the metabolism of cancer has been formed by the writings of those below, and from frequent discussions with a subset of them who have been more than generous with their time, especially Lewis Cantley (who led the team that discovered PI3K) and Dominic D’Agostino.
- Otto Warburg
- Lewis Cantley
- Dominic D’Agostino
- Craig Thompson
- Thomas Seyfried
- Eugene Fine
- Richard Feinman (not to be confused with Richard Feynman)
- Rainer Klement
- Reuben Shaw
- Matthew Vander Heiden
- Valter Longo
Further reading
I do plan to continue exploring this topic, but for those of you who want to know more right now and/or for those of you with an appetite for depth, I recommend the following articles, some technical, some not, but all worth the time to read. This is the short list:
- Relatively non-technical review article on the Warburg Effect written by Vander Heiden, Thompson, and Cantley
- Science piece written about cancer (for non-technical audience) by Gary Taubes
- Non-technical talk by Craig Thompson
- Detailed review article by Tom Seyfried
- Review article on the role of carb restriction in the treatment and prevention of cancer
- Talk given by author of above paper for those who prefer video
- Moderately technical review article by Shaw and Cantley
- Clinical paper on the role of metformin in breast cancer by Ana Gonzalez-Angulo
- Mouse study by Dom D’Agostino’s group examining role of ketogenic diet and hyperbaric oxygen on a very aggressive tumor model
- Mechanistic study by Feinman and Fine assessing means by which acetoacetate (a ketone body) suppresses tumor growth in human cancer cell lines
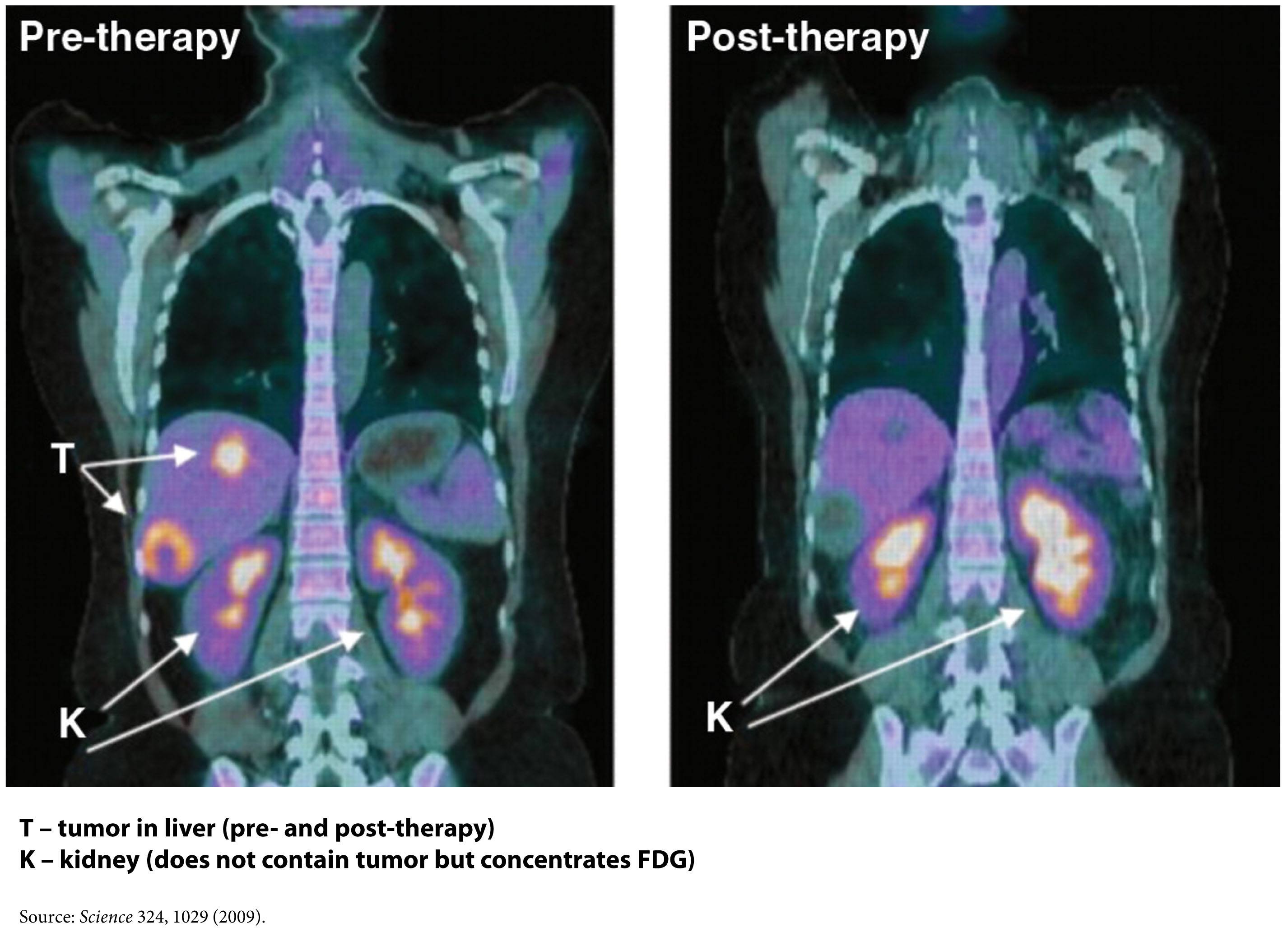
Figure of FDG-PET imaging showing no evidence of recurrent tumor after standard care treatment including a water-only fast and a ketogenic diet by Zuccoli et al., 2009 is licensed under CC by 2.0





Peter, thank you for a terrific overview, it answers nagging questions I’ve had ever since I started looking into ketogenic diet as adjuvant to chemotherapy (and even separately looked at hyperbaric oxygen treatment). I am very sad however, as it’s likely too late for my dad, he is recently hospitalized and has been given days-to-2weeks. However, he is not in much pain yet and a ‘hail mary’ attempt is not out of the question.
I am most interested in metformin at this point.
He has been ketogenic with great success during chemotherapy last year for the original colon cancer. His colon is clear. This was after three years of failed treatments. However the cancer had already metastasized to the liver and treatments since then seem to have made it very aggressive, the decline has in fact been dramatic in the last two months since a chemo-embolism treatment.
In my understanding, while trying to deliver chemo directly to the site is perhaps useful, doing the embolism part is the last thing anyone would want to do – cutting off oxygen is exactly what helps cancer cells resist chemo, isn’t it?
In any case, do you have any further information on the trials using metformin that you mentioned?
I would be very grateful for any links or names. I know this is asking much, but If it is at all possible for you to send them to me by email, I’d appreciate it – I am in transit to him right now and my email is easy to get, but a fast enough connection to load some web pages is not always available. I can then pass them on to someone who can look them up.
I had concluded a month ago that metformin might help, as it decreases the liver’s glucose production and since he’s been severely hypocaloric lately due to jaundice, cachexia and general malaise, those cancer cells have to be relying pretty exclusively on the liver’s gluconeogenesis.
Shutting off the spigot seems like a good idea.
Do you know if they can get glucose from anywhere else? Does there have to be no food at all in the digestive system, complete fasting, so that the colon microbiome shouldn’t be able to produce any either?
Any thoughts or ideas at all, much appreciated. And if none, thank you yet again for the state-of-the-art overview and the background links already provided. You do a public service.
Annique, I’m sorry to hear about your father. Unfortunately, the trials I know of investigating metformin are in the adjuvant setting (i.e., post surgical treatment, but with no evidence of tumor) or even the neoadjuvant setting (i.e., prior to first treatment). i’m not aware of stage IV trials. Sadly, it sounds like your father may be in an advanced ECOG stage for which there may not be as many opportunities for clinical trials. I’m sorry I can’t be of more help.
Peter, thank you very much for the reply and the insight.
Annique. I am a Stage IV mRCC (renal cell carcinoma) patient who convinced my oncologist to let me take Metformin (500mg ER) after I provided him with this research paper:
https://www.ncbi.nlm.nih.gov/pubmed/23773243
However, be aware that various kinds of tumors can “run” on fuels other than glucose. Some of them can ferment amino acids like glutamine. So restricting glucose is not a slam dunk. Still, Metformin is a very common drug given to Type II diabetics and it can’t hurt.
Yes, the Van Tulleken/BBC Horizon story was appalling. I actually contacted Dr Attia about it in a slight panic, which of course look silly now. Still, it would be great to hear any thoughts he has on the programme.
Peter, Though I am not a DR or very smart for that matter I have been digesting your writings and videos for the past 6 weeks as I have started a LCHF lifestyle trying to get healthy. Ironically I read this post yesterday and then last night I found out a customer of mine was just diagnosed with Stage 4 lung cancer. I do not know the type only that he has this late stage type of cancer. I am assuming that at this stage there is little conventionally that can be done that bears any form of true optimism to arresting this disease. I know it is difficult to have a lay person such as myself to offer advice (actually I might recommend a movie Cancer the Forbidden Cures) but not sure what he will be doing in the fight of his life. But based on your writings do you think cutting the sugar (carbs) from his diet would have any benefit, especially this late?
Either way I truly appreciate what you do and your delivery that allows the common man the ability to understand the complex.
Don
Don, lung cancer, in general, does not appear to be one of the cancers that is particularly sensitive to glucose, insulin, IGF, etc. So I’m not sure a reduction in sugar will have any effect, especially at an advanced stage. Possible, but I have no idea.
> Explanation 2: Cells become cancerous because they undergo some form of genetic insult.
What may be another hit on the somatic (gene) theory of cancer was just published.
“Premature Termination of Reprogramming In Vivo Leads to Cancer Development through Altered Epigenetic Regulation”
https://www.cell.com/retrieve/pii/S0092867414000154
The fulltext is paywalled, but the summary contains a provocative statement:
“We also demonstrate that iPSCs derived from Dox-withdrawn kidney tumor cells give rise to nonneoplastic kidney cells in mice, proving that they have not undergone irreversible genetic transformation.”
Currently, Monica Hughes, a PhD biologist, is searching for options and fighting for the treatment of her husbands’s glioblastoma. The story was recently posted at Free The Animal blog. Monica just posted her comment leading to their posts about the state and progress of treatment: https://freetheanimal.com/2014/02/medical-insurance-helping.html#comment-560143
Yes, I have connected with her and wish her and her husband my best.
Hello, One question bother me so much :” Why our stupid bodies prefer glucose if keton bodies seems to be so much healthier?”
Think of it in terms of flexibility and adaptability.
Yeap, I understand that but on the other hand even if we follow typical keto diet, we still cannot fall below certain level of glucose in our blood while it’s not difficult for our bodies to live without keton bodies.
.. but of course I am a layman, just a curious layman:)
and maybe our bodies just don’t behave in optimal way..sorry for spamming
There are at last appearing some really interesting and potentially fruitful new approaches to the understanding of cancer(s). Inter alia, there are proposals that cancer biochemistry is a very ancient phenomenon from an evolutionary point of view, dating back to times when this planet’s atmosphere was oxygen poor (why do animals, plants etc also develop cancers? – the ‘disease’ must have been present within the architecture of our evolutionary predecessors many millions of years ago).
If you even partially accept this way of looking at things, then the low-oxygen glucose mechanisms are not ‘stupid’, just a retrogressive switch, initiated by one or more stimuli, leading to a throw back to metabolic pathways that were ‘mainstream’ in earlier times.
See Paul Davies fascinating talk at https://www.youtube.com/watch?v=yoQYh0qPtz8
Peter, I just finished listening to your awesome ted talk. Thank you for your sharing your knowledge:)
Is it possible that higher authorities know this but do not disclose this because the financial shift this would cause? Lots of money treating cancer. Lots of money in agriculture. Not enough livestock.
I doubt it. Cognitive dissonance is a more powerful force than conspiracy.
I also just listened to your TED talk; it was wonderful, and I sent it to a doctor I know, an obese diabetic guy who is the epitome of the brilliant, kind, dedicated doctor.
After eating a fairly low carb diet for awhile, I’ve added more protein and way more fat recently. I lost 80 pounds over 25 years ago, and have maintained it, but now that I feel such incredible energy – normal energy – I realize I’d been underrating protein and of course fat all these years. (The low fat mainstream message has also subtly emphasized that we don’t need that much protein and I probably wasn’t eating more than 5 -6 ounces per day.) So, now I eat about 12 ounces of meat or fish per day and a lot of coconut oil. I just dump a big glob into my salad at night.
Just this past week I eliminated the last of the real carbs – 8 xylitol hard candies before bed, which totaled 40 grams of carbs. I had a headache for two days, which was no big deal. But here’s the problem – suddenly I’m not sleeping! I’ve always woken up a lot at night, but will fall right back to sleep. So, I get enough hours altogether. But now I’m really not sleeping. Do you know if this is a temporary situation they when I’m more adapted to ketosis will subside?
Thanks so much for what you’re doing.
Sounds like a bit of an adaptation period. Should pass.
Peter,
First, wonderful work. I learn a ton from you, and I appreciate your sophistication.
Second, I’ve had an interesting experience with ketosis that perhaps you or a knowledgable reader of yours can comment on.
I’m 29, very athlletic (7% BF, bench press 1.9x my weight, run 5:20 miles, 40 pullups, etc.), and have no concerns about bodyfat. I turned to keto because, 1. I’m a brain tumor survivor, and 2. not to be cliche, I wanted to be bulletproof. I’m a tech exec and need to be at the top of my game at all times. Your work and others made me aware of ketosis and the possible benefits it could provide for the mind & body. I therefore switched from a moderate fat, moderate protein (140 grams on 170 weight) veggie dense diet, which was about 30% carbs and working fine for me, to a VLC paleo diet. Here’s what happened, subjectively and objectively:
Objective Changes While in Ketosis:
-Lost 8 lbs, probably water; body fat may have crept up by a tiny bit, but that’s subjective (mirror assesments and pinching my abs)
-My LDL skyrocketed to 155, from around 100; HDL is 93.
-My triglycerides lowered to 70, from 85.
-My fasting glucose ROSE to 110-120, and my postprandial glucose always drops by 10+ points.
-My blood ketones were typically between .5 to 1.5
-My weight lifting decreased by about 15% on sets of 1-8 reps
Subjective:
-My focus was great during intermittant fasts – got a bit of a “high” from it, including some mental energy boosts. I didn’t really experience this to a discernable degree while in ketosis – though my ability to stay on task for hours at a time was definitely there.
-This is my biggest gripe: my memory sucked – I couldn’t keep track of details in conversations I had at work, and totally dropped the ball time and time again. It wasn’t caloric deprivation – I was consuming just as many as before, with tons of coconut butter, mana, MCT oil, grass fed liver, beef, pasture-raised eggs… the works. I also made sure to take in 5g+/- of sodium, and supplemented with iodine, selenium, magnesium, whey protein, B’s for my MTHFR c677t homozygous mutation, etc.
-My motivation declined. I felt less excitement for things – whether it be sex, work, achievement, going out at night, etc. I was flat, or emotionally dull.
-I may very well have had 0 instances of “staring off into the distance” to regain my thoughts over the course of the 5 months in ketosis.
-Reintroducing carbs made me more easily distracted (bad), but more energized, excitable and most importantly, my memory returned (great!).
MY QUESTION:
Does it sound like I may be genetically or epigenetically (especially in consideration of my anaerobic muscles’ needs) tuned to needing higher levels of clean carbs, and that ketosis may not be right for my brain? Fasting blood glucose going up, memory deficits and motivation issues all while in ketosis would seem to indicate such, but I’d love a professional’s opinion before I give up on ketosis. Or, more generally speaking, you may be more comfortable answering: Do you believe that some people, for whatever the reason, may be worse off while in ketosis (mentally, at least), and stand to benefit from 100+ grams of carbs /day?
Thanks so much!
The fasting glucose you can possibly ignore, its called Randle effect and seems to go up on VLC, but it is believed to not equiate to higher glucose uptake in the cells, no problem here.
Are you by chance APO e4 homozygote?
Did you supplment DHA?
Did you stop veggies entirely?
Did you consider doing just moderate aerobic workout? (instead of intense)
Did you try increasing sleep to 8-10 hours?
Did you supplement methylfolate and methylcobalamin?
How was your stress level?
How much carbohydrates did you reintroduce? Are you still able to maintain ketosis?
Did you supplement Potassium?
How many days a week did you work out?
Did you get your actual T3,T4 levels, for some Iodine is not enough?
Did you get Testosterone and Estradiol measured?
What IF regime did you do? Did you do ketogenic ad libitum + IF? How exactly?
There are a couple of possible theories to explore as you are doing just about everything.
All in all this is a pretty intense regime and you might be overdoing it. KD + IF is not so well explored as a maintenance diet. I do KD-R + fasting 2x a year for prevention and only if I dont have any stress. I read somewhere that Fasting + Stress is a pretty bad idea and might lead to adrenal fatigue, which might explain some of your symptoms.
Even though you were in ketosis, some part of your lifestlye might give you high ROS and impact your hippocampus (memory).
My best bet to an answer: You are overdoing one of the things you do (workout, fasting, nutrition…) or underoing (sleep, meditation…) others it in a certain way that limits some pathway producing some hormone(s) or increasing ROS, that apparently a glucose based pathway can produce or improve (given that you really didnt change anything else), affecting memory and motivation. We are all so different and you are also a special with the c677t and god knows what other variants.
In brief: Yes, some people, with some lifestyle factors & environment & genes, my not be able to do VLC as simple as others.
Key ideas:
How quickly can you reproduce the memory problems? If you could try again, would be interesting if NAC and ALA supplementation helps (check ROS theory).
If you are not supplementing 1g DHA, that would be another thing to try.
Potassium could contribute.
T3 could be a problem, I would have tried supplementation (careful) based on blood labs. Workout less.
High cortisol, low testosterone. Remove stress, reduce workout, dont fast while stressed or recovering from workout.
Hi Andre,
Thanks so much for your response. Here are my answers to your questions:
Are you by chance APO e4 homozygote? No I am not.
Did you supplement DHA? Yes – 800mg DHA (400mg EPA) /day + 1 krill oil. I also eat a fair amount of low mercury seafood.
Did you stop veggies entirely? No – I ate tons of veggies (5-10 servings of greens /day), while remaining in ketosis.
Did you consider doing just moderate aerobic workout? (instead of intense) Yes – I actually dropped my aerobic down to almost nothing, and only did 4 days/week of weightlifting (from 5-6 days weightlifting and 3 days cardio for the last 15+ years)
Did you try increasing sleep to 8-10 hours? Yes – tried for 8+ hours, but always found myself naturally waking up after 7. With moderate carb (150 grams), I can easily sleep for 8-9 hours, and have a little more trouble getting out of bed in the morning than when I’m in ketosis or low carb.
Did you supplement methylfolate and methylcobalamin? Yes (Homocystex Plus and Methyl B-12 5,000, sublingually).
How was your stress level? Moderate (physical, mental, emotional), although I feel like I got nervous / stressed a little more easily while in ketosis than moderate carb.
How much carbohydrates did you reintroduce? Are you still able to maintain ketosis? I’m at 150+ grams/day right now. I have purposely taken myself out of ketosis, as I felt it was negatively impacting my job performance. The interesting thing is that, anecdotally speaking, I feel I was able to focus for longer periods while in ketosis, but my memory suffered terribly. I also feel more optimistic and my libido has increased, after reintroducing carbs. I do feel more energy fluctuations with the carbs (more energized at times, but also more tired at times).
Did you supplement Potassium? Yes. 1 pill /night.
How many days a week did you work out? 4x, from formerly doing 6-7.
Did you get your actual T3,T4 levels, for some Iodine is not enough? No. Though, I do take iodoral (1x 12.5mg) /day.
Did you get Testosterone and Estradiol measured? No.
What IF regime did you do? Did you do ketogenic ad libitum + IF? How exactly? Ketosis, then 1-2x /wk IF via one serving of BP coffee (MCT, butter) + BCAAs in the morning, for a total fast of 16-20 hours.
Re: adrenal fatigue & stress, it’s definitely possible. I tried some adrenal support formulas, which included rhodiola & other adaptogens. I know adrenal fatigue is a complex (and controversial) matter – but to that point, if ketosis was in fact causing adrenal fatigue (and the list of symptoms I experienced), and adding moderate carbs back in brought me to a good place… I naturally feel inclined to stick with the carbs.
Re: overdoing/under doing something, that’s certainly possible. In regards to sleep, if I was under doing it, it was only because ketosis prevented me from staying asleep for as long as I wanted to (even with the addition of melatonin, gaba & magnesium). It also reduced my sleep efficiency (less time in deep & REM stages). Regarding workouts & fasting, I reduced my workouts to the lowest levels in 10+ years, and only fasted 1-2x /wk, on non-workout days.
This week I’m going to stick with carbs, but next week I will try ketosis again and see if I can reproduce the memory issues again. Regarding your suggestion and the ROS suspicion, I am already taking NAC + ALA (as well as ALCAR).
Having done both (ketogenic & moderate carb), and observing the differences, it seems like I’m much better suited for moderate carb, despite all of the promises of ketosis. I have the discipline and desire to experience ketosis & its benefits, but even with very careful experimentation, supplementation, etc., ketosis doesn’t seem to work for me and my memory, optimism, libido & excitement / passion. (Not to mention, my bench press increasing from 270 to 305 lbs within just 2 hours of 2 cups of ice cream, after 4 months of stagnation in ketosis! 🙂
Christ, you are tracking and taking more stuff than I do 🙂
Get your T3,T4, TSH levels now and once you try it next time every week. For some this gets disturbed on low-carb and that can explain quite some of the problems. Probably you are already in a very quiet and dark environment (I sleep with earplugs and face mask), so you can sleep as long as you need to?
Well, next time try it with less exercise and 8h sleep. Install f.lux on your machine and reduce blue ligth in the evening to make sure your circadium rhythm is not disturbed.
Your peak performance will suffer unless probably you try those super starches Peter uses. I dont train at peak.
For some also the adaption phase is quite long and difficult if not supplemented with loads of extra salt, magnesium, potassium, up to 6 weeks. For those actually a continous vs drastic reduction of sugar might be better.
If its not the thyroid and sleep doesnt help, then yes, there is another probably currently unknown probably genetic factor that will prevent you from going into deep ketosis with adverse effects.
If that memory effect is reproducible I would try an enormous amoung of MCT oil and see if that counters its effects (giving you brain mitochondria some extra energy).
Actually my apo e4 question was meant the other way. Most e4 I have encountered thrive on low-carb. So odds are you are e2 and e3.
One more thing, there are studies showing you should not fast when stressed, it could send your body & mind into hibernate mode (decreased libido and all that bunch). Thats why I am not a fan of IF, but rather KD-R and some extensive fast all couple of months…
In summary try
– have good sleep & stress level before you start
– dont workout that much, I run 4x a week max, even fasted, with intermittent sprints + some moderate muscle
work
– do not fast initially
If it fails
– double DHA
– triple MCT and other saturated fats
In any case get your t3,t4,tsh levels, beta-ketones and blood sugar as well.
post results!
Thanks so much.
Dr, Attia,
I am a Type II Diabetic who transitioned to a low carb diet (Atkins) 1 Jan. 2013. At the time I weighed more than 292 Lbs and had an A1C of 8.6. I was taking the maximum dosage of Actos Plus Met. Since that time I started an exercise program as well as continued with the diet. I have lost 62 Lbs and eliminated the Actos from my meds and reduced my metformin to 2000 mg a day. My A1C is now 6.3. In your writings you have mentioned several times the effects of metformin on the body. In this blog you alluded to several things it does but only detailed a single item. Would you consider writing a blog on this topic so those of us who take the drug can better understand its effects on our bodies as well as its potential effects on athletic performance? I just crossed over 1 million meters rowed and have had some issues in training that I would like to understand better if they are being affected by my metformin. I am hoping to eventually get off this drug as well. Thank you in your efforts to share this type of information with lay people. I wish you the very best in your pursuit to become the best Dr. you are able.
Impressive progress, Duffy. I have no idea if metformin is harming your progress. In theory it’s possible, if it’s preventing your liver from exporting glucose at the moments of “top end” power, but that’s never been studied to my knowledge, so at best it’s a mechanistic guess.
Dr. Attia,
Thank you for the kind words of encouragement. Could you consider writing a blog detailing all metformin does or is theorized to do in the body?
Knowing that CHO, Insulin, IGF impacts most cancers we can avoid this with VLC.
We also know that blood glucose under a VLC diet seems to increase slightly (assuming not overweight good health),
Thus, are the study results that blood glucose below 100 up to 75 decreases our risk for disease irrelevant for us fat-burners (Randle effect, blood glucose not equal glucose uptake) and those only apply to carbo burners? Or should we strive to reduce blood glucose below 100 under VLC and if so, how?
This is espeically intersting, nowing that some cancers can thrive even on low blood sugar and extract what they need, can they?
I am VLC for over a year, with daily moderate exercise and I hit 95 fasting glucose, 5% hba1c, unmeasurable CRP, ok fructosamine.
It’s not year clear to me, based on the limited data and mechanistic understanding, whether glucose removal matters more or less than ketone addition in some of these cancer models. My gut tells me any dietary strategy to cancer (treatment, not prevention, which is another story) will still require adjuvants, such as hyperbaric oxygen and/or chemo.
Peter, actually I was thinking about prevention only. Being VLC should we minimize further among where it naturally seems to flow and do we have any blood lab we can use ? (Is blood glucose still useful, including fructosamine, hba1c?)
Assume VLC and a healthy, low-toxin etc lifestlye, Insulin and IGF minimized, Ketones up to 1mmol. What then remains is glucose supply for cancer cells, that I would like to understand if there is need/use to minimize this further (from around 100). However, according to Randle, blood glucose increases on VLC playing against me.
Should we on VLC – being fat-adapted and likely keto-adapted – try to decrease blood sugar to the 75 level that is suggested by some big studies (not differentiating VLC dieters)? Because VLC gives me something around 95-105, to down that further would probably require ketones of 4mmol and stuff that impacts glucogenesis? Or just leave it float around there and relax? Do we know?
Hba1c is not useful due to the longer lifespan of in healthy people?
Blood glucose is not useful as it cant measure glucose uptake (Randle)
Fructosamine then?
Do we have any tests to validate how we are giving disadvantage to cancer cells?
All good questions. Most I don’t know the answers to, though.
Dr. Attia,
First of all I woudl like to thank you for the time and effort you invest in posting these great articles on your blog!
Second, I have a question about getting my ketone/glucose ratio at or above 1 (in light of advancing metastatic cancer).
I eat a dairy-free (except for butter) ketogenic diet, keep my carbs below 20g/day (purely from non-starchy veggies). Protein around 0.8g/kg bodyweight/day and fats coming from nuts (mainly macamamia, almonds), avocado, olive oil, butter and coconut oil and limited amounts of 85% dark chocolate. Doing this I have only been succesful at getting K/G >1 twice (ketones 4.4 and 4.9, glucose 2.6 and 3.1). Both times by restricting both calories (1000kcal/day) as well as protein (a little below 0.8g/kg bodyweight/day) to a low leveI. Both times I felt fine, except for hunger and some lightheadedness.
My question to you is, can you recommend any products to me that would help me get a sustained K/G ratio above 1 as advised by Dr. Seyfried, seen as a KD-R diet alone usually gets me only a K/G around 0.5.
Thanks so much!
Not in general, no. Very person specific. Plus, for many this requires some combination of the following: caloric restriction, MCT, exogenous ketones.
The exogenous ketones was actually what I was referring to. All I can find thus far is Ketoforce (have not tried this yet). Any similar/better synthetic ketone products you know of that could be of help to me?
To you knowledge, does taking synthetic ketones only increase circulating ketones or could it also help push blood glucose from a ‘normal’ fasting range to about 2.5 – 3 mmol/l?(perhaps by indirectly limiting need for gluconeogenesis?)
Several variants should be on the market this year.
Thoughts?
Biomarker Profiling by Nuclear Magnetic Resonance Spectroscopy for the Prediction of All-Cause Mortality: An Observational Study of 17,345 Persons
https://www.plosmedicine.org/article/info%3Adoi%2F10.1371%2Fjournal.pmed.1001606
Applies to both cardiac and cancer deaths.
I don’t think it tells us much beyond the association. Certainly contains no causal info.
I am 42 y old. Male. I am 1.8m and 74kg 9-10% body fat. I race bicycles. Wanted to go down 4-5kg and my dietitian said ‘try low carb’
I did. I thought I give it a try…
I also did a lot of reading.
I started 01.01.14 – 40g carbs a day. For the last 9 days (very similar to last weeks) I ate 2.2g fat per kg body, 1.6g protein.
I feel very well. Last Sat did a 5h ride on a bike, 3500kcal with some 20-50min heavy going (270NP, which is quite hard for me). No carb consumed dring or prior to the ride. No energy dips felt at all. Actually felt strong…
Had a blood test yesterday. Fasting glucose and cortisol came out bad.
How come there is so little mention of low carb high cortisol relationship on your blog…? In fact when one does a search for cortisol on the blog it comes out in four posts and no proper treatment, no mention…
My results
LDL (not calculated) 107
LDL calculated 79 (funny, I though, heaving read all the web stuff, that it would be other way round, though both within the norm)
HDL 101
Trigri 50
Glucose 99.6
HbA1c 5.1
Cortisol 792 (normal range 171-536)
Testosteron 23.2
What am I doing wrong? I am sure I cannot be alone in the above position. How many people test their cortisol levels..?
So let me get this straight, you feel great, but your am cortisol is a bit high? What is your question/concern? (I’m not looking for the response, “My cortisol is too high.”)
Hi,
Apologies I have not been more specific.
I have a problem with:
1. Higher level of glucose (top end of the normal range)
2. Very high level of cortisol and effect it can have if remains that way for long
3. No drop in weight I was 74-75kg in Dec 2013 and still am the same weight today.
I understand that cortisol is released to mobilize amino acids from tissues to increase the availability of glucose through gluconeogenesis. The less glycogen we have stored the more pronounced cortisol release. This especially is true during high intensity long training sessions (like my cycling, 5-6h with 2-3 of hard riding or just 1-2h hard training session). I also do two times a week some weights and cross training to keep up overall body strength (this is 500-700kcal per h sessions).
If the above is true, I would suspect that LC diets result in an increased exercise-induced cortisol response.
I have been consuming c 1.6-1.7g of protein per day for the last 8 weeks This is probably too much as it should have been c 1g (per kg of body mass).
Is it fair to assume that this excess protein was converted into glucose via gluconeogenesis? Hence elevated glucose levels and no weight loss (my body was burning those proteins and not fat)
The thing is that from what I read, had I consumed say 1g of protein (so much less) my body would still use proteins to synthesise glycogen (thanks to elevated cortisol levels, which in turn is a result of low glycogen levels in my body and heavy training)??
Elevated cortisol levels can result from various factors: low carb diet, heavy aerobic training, too much protein, stress, too much caffeine (others?).
In my case at least three played a role (first three).
I understand that for somebody who is overweight and does little to no exercise going low carb with appropriate level of protein (not too much) the cortisol response may not be as big hence his/her body indeed accesses lipids as source of energy, hence weight loss occurs as expected.
In my case I have recorded zero weight loss. FYI my daily calorie intake for the last 9 days was 2300kcal. Earlier than those 9 days it was c 2000kcal which was too low.
Should I be supplementing with say UCAN around my training and how much? 30g pre session and 30g during plus 30g UCAN protein enhanced after?
Many thx Peter
It would be great to see some comments from other endurance athletes.
Przemek
Please see the latest video from Dr Lustig on the weight issue. It could be that the decresed intake of glucose is resulting in an increased amount of muscle mass and less fatt mass. As Lustig says the scales do not tell you anything. Fat Chance 2.0 is the video.
Dear Dr Attia,
what is your opinion on Polly Matzinger’s Danger Model of immunity? Here is for instance a very informative radio interwiev with her from 2010, also about Coley’s toxins that you mentioned and cancer treatment (1 hour long, sorry):
https://www.peoplespharmacy.com/2010/08/07/751-coleys-toxin-cancer-and-immunology/
“Dr. William Coley was a cancer surgeon at the turn of the 20th century. In an effort to improve the treatment he could offer his patients, he created a toxin that made them really sick. If they recovered from their fever, however, they were often cured of their sarcomas.
A century later, cancer researchers are taking a new look at Coley’s toxin and how it might help us understand spontaneous remissions and the role of the immune system. In exploring this topic, we encounter an innovative immunologist who has developed a new paradigm for how the immune system works.”
Read “A commotion in the blood” and “The transformed cell”
— Read “A commotion in the blood” and “The transformed cell”
——————————————————————
Thank you, it definitely sounds like good reading. However, “The transformed cell” is from 1992, Matzinger published her Danger Model in 1994 or so. Here in the above mentioned interwiev in 37:35 (file MatzingerFull) she tells what was according to her wrong in Rosenberg’s approach.
And here it is again from her talk UCD Dublin (February-March 2012) in 32:30 – 37 min
https://youtu.be/1eSiAUHDhyA?t=32m30s
Coincidentally, my son is currently in bed with very high fever and Streptocosus pyogenes thriving in his throat (and penicillin, of course, I am not risking anything). But I told him that for consolation that “every cloud has a silver lining”.