One night, as I alluded to in this post, Tim and I were having dinner and the topic of cancer came up. Personally and professionally I have a great interest in cancer, so when Tim asked if I could write something about cancer that was: (i) interesting to a broad audience, (ii) not technically over the top, (iii) not my typical 5,000 word dissertation, (iv) yet nuanced enough for his readers, I agreed to give it a shot, in about 1,000 words. (The content of this blog went up on Tim’s blog last week, but I’ve reproduced it here, less Tim’s commentary.)
Semantics and basics
Before jumping into this topic I want to be sure all readers — regardless of background — have a pretty good understanding of the ‘basics’ about cancer and metabolism. In an effort to do this efficiently, I’ll list concepts here, such that folks can skip them if they want to, or refer back as necessary. This way, I don’t need to disrupt the ‘story’ with constant definitions. (Yes, I realize this is sort of cheating on my 1,000 word promise.)
Cancer – a collection of cells in our bodies that grow at roughly normal speeds, but that do not respond appropriately to cell signaling. In other words, while a collection of ‘normal’ cells will grow and stop growing in response to appropriate messages from hormones and signals, cancer cells have lost this property. Contrary to popular misconception, cancers cells do not grow especially fast relative to non-cancer cells. The problem is they don’t ‘know’ when to stop growing.
Metabolism – the process of converting the stored energy in food (chemical energy contained mostly within the bonds of carbon and hydrogen atoms) into usable energy for the body to carry out essential and non-essential work (e.g., ion transport, muscle contraction).
ATP – adenosine triphosphate, the ‘currency’ of energy used by the body. As its name suggests, this molecule has three (tri) phosphates. Energy is liberated for use when the body converts ATP to ADP (adenosine diphosphate), by cutting off one of the phosphate ions in exchange for energy.
Glucose – a very simple sugar which many carbohydrates ultimately get broken down into via digestion; glucose is a ring of 6-carbon molecules and has the potential to deliver a lot, or a little, ATP, depending on how it is metabolized.
Fatty acid – the breakdown product of fats (either those stored in the body or those ingested directly) which can be of various lengths (number of joined carbon atoms) and structures (doubled bonds between the carbon atoms or single bonds).
Aerobic metabolism – the process of extracting ATP from glucose or fatty acids when the demand for ATP is not too great, which permits the process to take place with sufficient oxygen in the cell. This process is highly efficient and generates a lot of ATP (about 36 units, for example, from one molecule of glucose) and easy to manage waste products (oxygen and carbon dioxide).
The process of turning glucose and fatty acid into lots of ATP using oxygen is called ‘oxidative phosphorylation.’
Anaerobic metabolism – the process of extracting ATP from glucose (but not fatty acids) when the demand for ATP is so great that the body cannot deliver oxygen to cells quickly enough to accommodate the more efficient aerobic pathway. The good news is that we can do this (otherwise a brief sprint, or very difficult exertion would be impossible). The bad news is this process generates much less ATP per carbon molecule (about 4 units of ATP per molecule of glucose), and it generates lactate, which is accompanied by hydrogen ions. (Contrary to popular belief, it’s the latter that causes the burning in your muscles when you ask your body to do something very demanding, not the former).
Mitochondria – the part of the cell where aerobic metabolism takes place. Think of a cell as a town and the mitochondria as the factory that converts the stored energy into usable energy. If food is natural gas, and usable energy is electricity, the mitochondria are the power plants. But remember, mitochondria can only work when they have enough oxygen to process glucose or fatty acids. If they don’t, the folks outside of the factory have to make due with suboptimally broken down glucose and suboptimal byproducts.
DNA – deoxyribonucleic acid, to be exact, is the so-called “building block” of life. DNA is a collection of 4 subunits (called nucleotides) that, when strung together, create a code. Think of nucleotides like letters of the alphabet. The letters can be rearranged to form words, and words can be strung together to make sentences.
Gene – if nucleotides are the letters of the alphabet, and DNA is the words and sentences, genes are the books – a collection of words strung together to tell a story. Genes tell our body what to build and how to build it, among other things. In recent years, scientists have come to identify all human genes, though we still have very little idea what most genes ‘code’ for. It’s sort of like saying we’ve read all of War and Peace, but we don’t yet understand most of it.
FDG-PET – a type of ‘functional’ radiographic study, often called a ‘pet scan’ for short, used to detect cancer in patients with a suspected tumor burden (this test can’t effectively detect small amounts of cancer and only works for ‘established’ cancers). F18 is substituted for -OH on glucose molecules, making something called 2-fluoro-2-deoxy-D-glucose (FDG), an analog of glucose. This molecule is detectable by PET scanners (because of the F18) and shows which parts of the body are most preferentially using glucose.
Phosphoinositide 3-kinase – commonly called PI3K (pronounced “pee-eye-three-kay”), is an enzyme (technically, a family of enzymes) involved in cell growth and proliferation. Not surprisingly, these enzymes play an important role in cancer growth and survival, and cancer cells often have mutations in the gene encoding PI3K, which render PI3K even more active. PI3Ks are very important in insulin signaling, which may in part explain their role in cancer growth, as you’ll see below.
The story (in about 1,000 words, as promised)
In 1924 a scientist named Otto Warburg happened upon a counterintuitive finding. Cancer cells, even in the presence of sufficient oxygen, underwent a type of metabolism cells reserved for rapid energy demand – anaerobic metabolism. In fact, even when cancer cells were given additional oxygen, they still almost uniformly defaulted into using only glucose to make ATP via the anaerobic pathway. This is counterintuitive because this way of making ATP is typically a last resort for cells, not a default, due to the very poor yield of ATP.
This observation begs a logical question? Do cancer cells do this because it’s all they can do? Or do they deliberately ‘choose’ to do this? I’m not sure the answer is entirely clear or even required to answer the macro question I’ve posed in this post. However, being curious people we like answers, right?
The first place to look is at the mitochondria of the cancer cells. Though not uniformly the case, most cancers do indeed appear to have defects in their mitochondria that prevent them from carrying out oxidative phosphorylation.
Explanation 1
Cancer cells, like any cells undergoing constant proliferation (recall: cancer cells don’t stop proliferating when told to do so), may be optimizing for something other than energy generation. They may be optimizing for abundant access to cellular building blocks necessary to support near-endless growth. In this scenario, a cancer would prefer to rapidly shuttle glucose through itself. In the process, it generates the energy it needs, but more importantly, it gains access to lots of carbon, hydrogen, and oxygen atoms (from the breakdown of glucose). The atoms serve as the necessary input to the rate-limiting step of their survival — growth. The selection of cancer cells is based on this ability to preferentially grow by accessing as much cellular substrate as possible.
Explanation 2
Cells become cancerous because they undergo some form of genetic insult. This insult – damage to their DNA – has been shown to result in the turning off of some genes (those that suppress tumor growth) and/or the activation of other genes (those that promote cell growth unresponsive to normal cell-signaling). Among other things, this damage to their DNA also damages their mitochondria, rendering cancer cells unable to carry out oxidative phosphorylation. So, to survive they must undergo anaerobic metabolism to make ATP.
Whichever of these is more accurate, the end result appears the same – cancer cells almost exclusively utilize glucose to make ATP without the use of their mitochondria. A detailed discussion of which explanation is better is beyond the scope of my word allotment, and it’s not really the point I want to make. The point is, cancer cells have a metabolic quirk. Regardless of how much oxygen and fatty acid they have access to, they preferentially use glucose to make ATP, and they do it without their mitochondria and oxygen.
So, can this be exploited to treat or even prevent cancer?
One way this quirk has been exploited for many years is in medical imaging. FDG-PET scans are a useful tool for non-invasively detecting cancer in people. By exploiting the obligate glucose consumption of cancer cells, the FDG-PET scan is a powerful way to locate cancer (see figure).
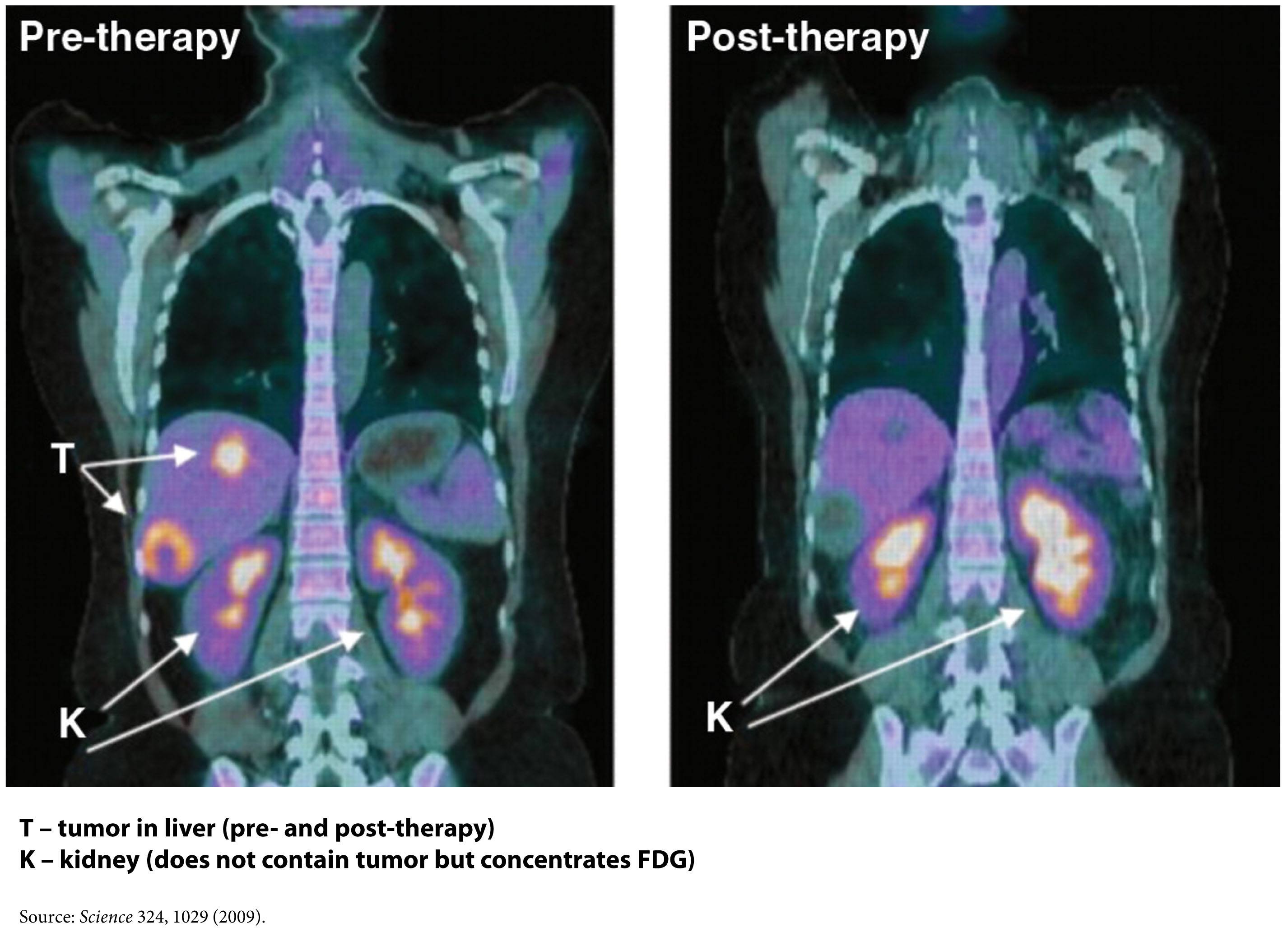
You can probably tell where I’m leading you. What happens if we reduce the amount of glucose in the body? Could such an intervention ‘starve’ cancer cells? An insight into this came relatively recently from an unlikely place – the study of patients with type 2 diabetes.
In the past few years, three retrospective studies of patients taking a drug called metformin have shown that diabetic patients who take metformin, even when adjusted for other factors such as body weight and other medications, appear to get less cancer. And when they do get cancer, they appear to survive longer. Why? The answer may lie in what metformin does. Metformin does many things, to be clear, but chief among them is activating an enzyme called AMP kinase, which is important in suppressing the production of glucose in the liver (the liver manufactures glucose from protein and glycerol and releases it to the rest of the body). This drug is used in patients with diabetes to reduce glucose levels and thereby reduce insulin requirement.
So, the patients taking metformin may have better cancer outcomes because their glucose levels were lower, or because such patients needed less insulin. Insulin and insulin-like growth factor (IGF-1) also appear to play an integral role in cancer growth as recently demonstrated by the observation that people with defective IGF-1 receptors appear immune to cancer. Or, it may be that activation of AMP kinase in cancer cells harms them in some other way. We don’t actually know why, but we do know that where there is smoke there is often fire. And the ‘smoke’ in this case is that a relatively innocuous drug that alters glucose levels in the body appears to interfere with cancer.
This may also explain why most animal models show that caloric restriction improves cancer outcomes. Though historically, this observation has been interpreted through the lens of less ‘food’ for cancer. A more likely explanation is that caloric restriction is often synonymous with glucose reduction, and it may be the glucose restriction per se that is keeping the cancer at bay.
Fortunately this paradigm shift in oncology – exploiting the metabolic abnormality of cancer cells – is gaining traction, and doing so with many leaders in the field.
Over a dozen clinical trials are underway right now investigating this strategy in the cancers that appear most sensitive to this metabolic effect – breast, endometrial, cervical, prostate, pancreatic, colon, and others. Some of these trials are simply trying to reproduce the metformin effect in a prospective, blinded fashion. Other trials are looking at sophisticated ways to target cancer by exploiting this metabolic abnormality, such as targeting PI3K directly.
To date, no studies in humans are evaluating the therapeutic efficacy of glucose and/or insulin reduction via diet, though I suspect that will change in the coming year or two, pending outcomes of the metformin trials.
Last point (beyond my 1,000 word allotment)
Check out this blast from the past! Gary Taubes, who is currently working hard on his next book, came across the article the other day from 1887.

Influences
I’ve been absurdly blessed to study this topic at the feet of legends, and to be crystal clear, not one thought represented here is original work emanating from my brain. I’m simply trying to reconstruct the story and make it more accessible to a broader audience. Though I trained in oncology, my research at NIH/NCI focused on the role of the immune system in combating cancer. My education in the metabolism of cancer has been formed by the writings of those below, and from frequent discussions with a subset of them who have been more than generous with their time, especially Lewis Cantley (who led the team that discovered PI3K) and Dominic D’Agostino.
- Otto Warburg
- Lewis Cantley
- Dominic D’Agostino
- Craig Thompson
- Thomas Seyfried
- Eugene Fine
- Richard Feinman (not to be confused with Richard Feynman)
- Rainer Klement
- Reuben Shaw
- Matthew Vander Heiden
- Valter Longo
Further reading
I do plan to continue exploring this topic, but for those of you who want to know more right now and/or for those of you with an appetite for depth, I recommend the following articles, some technical, some not, but all worth the time to read. This is the short list:
- Relatively non-technical review article on the Warburg Effect written by Vander Heiden, Thompson, and Cantley
- Science piece written about cancer (for non-technical audience) by Gary Taubes
- Non-technical talk by Craig Thompson
- Detailed review article by Tom Seyfried
- Review article on the role of carb restriction in the treatment and prevention of cancer
- Talk given by author of above paper for those who prefer video
- Moderately technical review article by Shaw and Cantley
- Clinical paper on the role of metformin in breast cancer by Ana Gonzalez-Angulo
- Mouse study by Dom D’Agostino’s group examining role of ketogenic diet and hyperbaric oxygen on a very aggressive tumor model
- Mechanistic study by Feinman and Fine assessing means by which acetoacetate (a ketone body) suppresses tumor growth in human cancer cell lines