Want to catch up with other articles from this series?
- The straight dope on cholesterol – Part I
- The straight dope on cholesterol – Part II
- The straight dope on cholesterol – Part III
- The straight dope on cholesterol – Part IV
- The straight dope on cholesterol – Part V
- The straight dope on cholesterol – Part VI
- The straight dope on cholesterol – Part VII
- The straight dope on cholesterol – Part VIII
- The straight dope on cholesterol – Part IX
Previously, across 8 parts of this series we’ve laid the groundwork to ask perhaps the most important question of all:
What should you eat to have the greatest chance of delaying the arrival of cardiovascular disease?
Before we get there, since this series has been longer and more detailed than any of us may have wanted, it is probably worth reviewing the summary points from the previous posts in this series (or you can just skip this and jump to the meat of this post).
What we’ve learned so far
- Cholesterol is “just” another fancy organic molecule in our body but with an interesting distinction: we eat it, we make it, we store it, and we excrete it – all in different amounts.
- The pool of cholesterol in our body is essential for life. No cholesterol = no life.
- Cholesterol exists in 2 forms – unesterified or “free” (UC) and esterified (CE) – and the form determines if we can absorb it or not, or store it or not (among other things).
- Much of the cholesterol we eat is in the form of CE. It is not absorbed and is excreted by our gut (i.e., leaves our body in stool). The reason this occurs is that CE not only has to be de-esterified, but it competes for absorption with the vastly larger amounts of UC supplied by the biliary route.
- Re-absorption of the cholesterol we synthesize in our body (i.e., endogenous produced cholesterol) is the dominant source of the cholesterol in our body. That is, most of the cholesterol in our body was made by our body.
- The process of regulating cholesterol is very complex and multifaceted with multiple layers of control. I’ve only touched on the absorption side, but the synthesis side is also complex and highly regulated. You will discover that synthesis and absorption are very interrelated.
- Eating cholesterol has very little impact on the cholesterol levels in your body. This is a fact, not my opinion. Anyone who tells you different is, at best, ignorant of this topic. At worst, they are a deliberate charlatan. Years ago the Canadian Guidelines removed the limitation of dietary cholesterol. The rest of the world, especially the United States, needs to catch up. To see an important reference on this topic, please look here.
- Cholesterol and triglycerides are not soluble in plasma (i.e., they can’t dissolve in water) and are therefore said to be hydrophobic.
- To be carried anywhere in our body, say from your liver to your coronary artery, they need to be carried by a special protein-wrapped transport vessel called a lipoprotein.
- As these “ships” called lipoproteins leave the liver they undergo a process of maturation where they shed much of their triglyceride “cargo” in the form of free fatty acid, and doing so makes them smaller and richer in cholesterol.
- Special proteins, apoproteins, play an important role in moving lipoproteins around the body and facilitating their interactions with other cells. The most important of these are the apoB class, residing on VLDL, IDL, and LDL particles, and the apoA-I class, residing for the most part on the HDL particles.
- Cholesterol transport in plasma occurs in both directions, from the liver and small intestine towards the periphery and back to the liver and small intestine (the “gut”).
- The major function of the apoB-containing particles is to traffic energy (triglycerides) to muscles and phospholipids to all cells. Their cholesterol is trafficked back to the liver. The apoA-I containing particles traffic cholesterol to steroidogenic tissues, adipocytes (a storage organ for cholesterol ester) and ultimately back to the liver, gut, or steroidogenic tissue.
- All lipoproteins are part of the human lipid transportation system and work harmoniously together to efficiently traffic lipids. As you are probably starting to appreciate, the trafficking pattern is highly complex and the lipoproteins constantly exchange their core and surface lipids.
- The measurement of cholesterol has undergone a dramatic evolution over the past 70 years with technology at the heart of the advance.
- Currently, most people in the United States (and the world for that matter) undergo a “standard” lipid panel, which only directly measures TC, TG, and HDL-C. LDL-C is measured or most often estimated.
- More advanced cholesterol measuring tests do exist to directly measure LDL-C (though none are standardized), along with the cholesterol content of other lipoproteins (e.g., VLDL, IDL) or lipoprotein subparticles.
- The most frequently used and guideline-recommended test that can count the number of LDL particles is either apolipoprotein B or LDL-P NMR, which is part of the NMR LipoProfile. NMR can also measure the size of LDL and other lipoprotein particles, which is valuable for predicting insulin resistance in drug naïve patients, before changes are noted in glucose or insulin levels.
- The progression from a completely normal artery to a “clogged” or atherosclerotic one follows a very clear path: an apoB containing particle gets past the endothelial layer into the subendothelial space, the particle and its cholesterol content is retained, immune cells arrive, an inflammatory response ensues “fixing” the apoB containing particles in place AND making more space for more of them.
- While inflammation plays a key role in this process, it’s the penetration of the endothelium and retention within the endothelium that drive the process.
- The most common apoB containing lipoprotein in this process is certainly the LDL particle. However, Lp(a) and apoB containing lipoproteins play a role also, especially in the insulin resistant person.
- If you want to stop atherosclerosis, you must lower the LDL particle number. Period.
- At first glance it would seem that patients with smaller LDL particles are at greater risk for atherosclerosis than patients with large LDL particles, all things equal.
- “A particle is a particle is a particle.” If you don’t know the number, you don’t know the risk.
- With respect to laboratory medicine, two markers that have a high correlation with a given outcome are concordant – they equally predict the same outcome. However, when the two tests do not correlate with each other they are said to be discordant.
- LDL-P (or apoB) is the best predictor of adverse cardiac events, which has been documented repeatedly in every major cardiovascular risk study.
- LDL-C is only a good predictor of adverse cardiac events when it is concordant with LDL-P; otherwise it is a poor predictor of risk.
- There is no way of determining which individual patient may have discordant LDL-C and LDL-P without measuring both markers.
- Discordance between LDL-C and LDL-P is even greater in populations with metabolic syndrome, including patients with diabetes. Given the ubiquity of these conditions in the U.S. population, and the special risk such patients carry for cardiovascular disease, it is difficult to justify use of LDL-C, HDL-C, and TG alone for risk stratification in all but the most select patients.
- To address this question, however, one must look at changes in cardiovascular events or direct markers of atherosclerosis (e.g., IMT) while holding LDL-P constant and then again holding LDL size constant. Only when you do this can you see that the relationship between size and event vanishes. The only thing that matters is the number of LDL particles – large, small, or mixed.
- HDL-C and HDL-P are not measuring the same thing, just as LDL-C and LDL-P are not.
- Secondary to the total HDL-P, all things equal it seems smaller HDL particles are more protective than large ones.
- As HDL-C levels rise, most often it is driven by a disproportionate rise in HDL size, not HDL-P.
- In the trials which were designed to prove that a drug that raised HDL-C would provide a reduction in cardiovascular events, no benefit occurred: estrogen studies (HERS, WHI), fibrate studies (FIELD, ACCORD), niacin studies, and CETP inhibition studies (dalcetrapib and torcetrapib). But, this says nothing of what happens when you raise HDL-P.
- Don’t believe the hype: HDL is important, and more HDL particles are better than few. But, raising HDL-C with a drug isn’t going to fix the problem. Making this even more complex is that HDL functionality is likely as important, or even more important, than HDL-P, but no such tests exist to “measure” this.
Did you say “delay?”
That’s right. The question posed above did not ask how one could “prevent” or eliminate the risk cardiovascular disease, it asked how one could “delay” it. There is a difference. To appreciate this distinction, it’s worth reading this recent publication by Allan Sniderman and colleagues. Allan sent me a copy of this paper ahead of publication a few months ago in response to a question I had posed to him over lunch one day. I asked,
“Allan, who has a greater 5-year risk for cardiovascular disease, a 25 year-old with a LDL-P/apoB in the 99th percentile or a 75-year-old with a LDL-P/apoB in the 5th percentile?”
The paper Allan wrote is noteworthy for at least 2 reasons:
- It’s an excellent reminder that age is a paramount risk factor for cardiovascular disease.
- It provides a much better (causal) model for atherosclerosis than the typical age-driven models, and explains why age is an important risk factor.
What do I mean by this? Most risk calculators (e.g., Framingham) take their inputs (e.g., age, gender, LDL-C, HDL-C, smoking, diabetes, blood pressure) and calculate a 10-year risk score. If you’ve ever played with these models you’ll quickly see that age drives risk more than any other input. But why? Is there something inherently “risky” about being older?
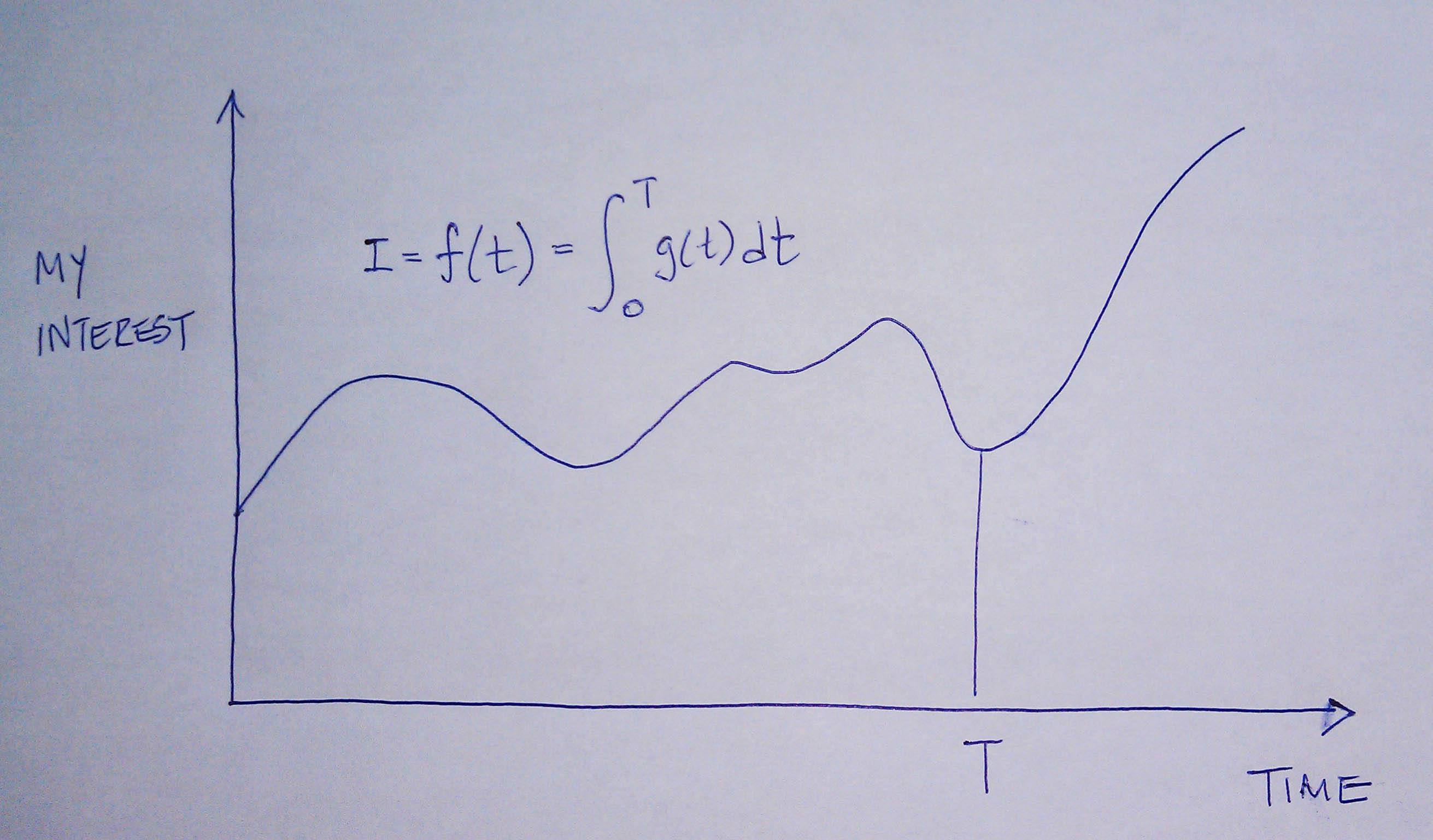
Sniderman and many others would argue (and I agree) that the reason age is a strong predictor of risk has to do with exposure to apoB particles — LDL, Lp(a), and apoB-carrying remnants. Maybe it’s because I’m a math geek, but such models just seem intuitive to me because I think of most things in life in terms of calculus, especially integrals, the “area under a curve.”
[I once tried to explain to a girlfriend who thought I wasn’t spending enough time with her that my interest in her should be thought of in terms of the area under the curve, rather than any single point in time. That is, think in terms of the integral function, not the point-in-time function. Needless to say, she broke up with me on the spot (in the middle of a parking lot!), despite me drawing a very cool picture illustrating the difference, which I’ve re-created, below.]
The reason age is such a big driver of risk is that the longer your artery walls are exposed to the insult of apoB particles, the more likely they are to be damaged, for all the reasons we covered in Part IV of this series. [This paper also reviews the clinical situation of PCSK9 mutations which builds a very compelling case for the causal model of apoB particles in the development of atherosclerosis].
What does eating have to do with cardiovascular risk?
So now that everyone is on the edge of their seat in anticipation of this punch-line, let me provide two important caveats.
First, there are no long-term studies – either in primary or secondary prevention – examining the exact question we all want to know the answer to with respect to the role of dietary intervention on cardiovascular disease. There are short-term studies, some of which I will highlight, which look at proxies for cardiovascular disease, but all of the long-term studies (looking at secondary prevention), are either drug studies or multiple intervention studies (e.g., cholesterol-lowering drug(s) + blood pressure reducing drug(s) + dietary intervention + exercise + …).
In other words, the “dream” study has not been done and won’t be done for a long time. The “dream” study would follow 2 randomized groups for many years and only make one change between the groups. Group 1 would consume a standard American diet and group 2 would consume a very-low carbohydrate diet. Furthermore, compliance within each group would be excellent (many ways to ensure this, but none of them are inexpensive – part of why this has not been done) and the study would be powered to detect “hard outcomes” (e.g., death), instead of just “soft outcomes” (e.g., changes in apoB, LDL-C, LDL-P, TG).
Second, everything we have learned to date on the risk relationship between cardiovascular disease and risk markers is predicated on the assumption that a risk maker of level X in a person on diet A is the same as it would be for a person on diet B.
Since virtually all of the thousands of subjects who have made up the dozens of studies that form the basis for our understanding on this topic were consuming some variant of the “standard American diet” (i.e., high-carb), it is quite possible that what we know about risk stratification is that this population is not entirely fit for extrapolation to a population on a radically different diet (e.g., a very-low carbohydrate diet or a ketogenic diet). Many of you have asked about this, and my comments have always been the same. It is entirely plausible that an elevated level of LDL-P or apoB in someone consuming a high-carb diet portends a greater risk than someone on a ketogenic or low-carb diet. There are many reasons why this might be the case, and there are many folks who have made compelling arguments for this hypothesis.
But we can’t forget the words of Thomas Henry Huxley, who said, “The great tragedy of science is the slaying of a beautiful hypothesis by an ugly fact.” Science is full of beautiful hypothesis slayed by ugly facts. Only time will tell if this hypothesis ends up in that same graveyard, or changes the way we think about lipoproteins and atherosclerosis.
The role of sugar in cardiovascular disease
Let’s start with what we know, then fill in the connections, with the goal of creating an eating strategy for those most interested in delaying the onset of cardiovascular disease.
There are several short-term studies that have carefully examined the impact of sugar, specifically, on cardiovascular risk markers. Let’s examine one of them closely. In 2011 Peter Havel and colleagues published a study titled Consumption of fructose and HFCS increases postprandial triglycerides, LDL-C, and apoB in young men and women. If you don’t have access to this journal, you can read the study here in pre-publication form. This was a randomized trial with 3 parallel arms (no cross-over). The 3 groups consumed an isocaloric diet (to individual baseline characteristics) consisting of 55% carbohydrate, 15% protein, and 30% fat. The difference between the 3 groups was in the form of their carbohydrates.
Group 1: received 25% of their total energy in the form of glucose
Group 2: received 25% of their total energy in the form of fructose
Group 3: received 25% of their total energy in the form of high fructose corn syrup (55% fructose, 45% glucose)
The intervention was relatively short, consisting of both an inpatient and outpatient period, and is described in the methodology section.
Keep in mind, 25% of total energy in the form of sugar is not as extreme as you might think. For a person consuming 2,400 kcal/day this amounts to about 120 pounds/year of sugar, which is slightly below the average consumption of annual sugar in the United States. In that sense, the subjects in Group 3 can be viewed as the “control” for the U.S. population, and Group 1 can be viewed as an intervention group for what happens when you do nothing more in your diet than remove sugar, which was the first dietary intervention I made in 2009.
Despite the short duration of this study and the relatively small number of subjects (16 per group), the differences brought on by the interventions were significant. The figure below shows the changes in serum triglycerides via 3 different ways of measuring them. Figure A shows the difference in 24-hour total levels (i.e., the area under the curve for serial measurements – hey, there’s our integral function again!). Figure B shows late evening (post-prandial) differences. Figure C shows the overall change in fasting triglyceride level from baseline (where sugar intake was limited for 2 weeks and carbohydrate consumption consisted only of complex carbohydrates).
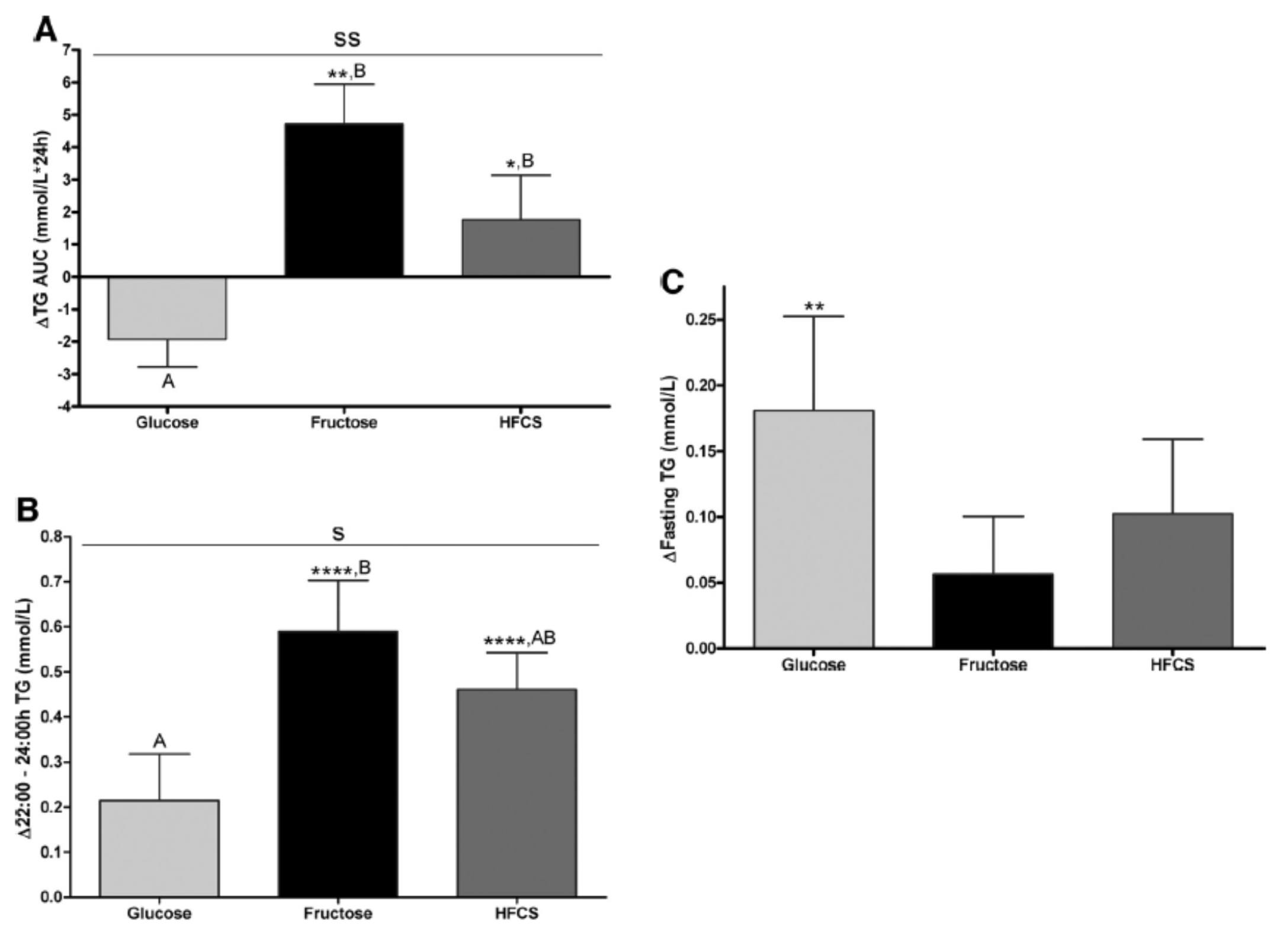
The differences were striking. The group that had all fructose and HFCS removed from their diet, despite still ingesting 55% of their total intake in the form of non-sugar carbohydrates, experienced a decline in total TG (Figure A, which represents the daily integral of plasma TG levels, or AUC). However, that same group experienced the greatest increase in fasting TG levels (Figure C). Post-prandial TG levels were elevated in all groups, but significantly higher in the fructose and HFCS groups (Figure B). The question this begs, of course, is which of these measurements is most predictive of risk?
Historically, fasting levels of TG are used as the basis of risk profiling (Figure C), and according to this metric glucose consumption appears even worse than fructose or HFCS. However, recent evidence suggests that post-prandial levels of TG (Figure B) are a more accurate way to assess atherosclerotic risk, as seen here, here, and here. One question I have is why did the AUC calculations in Figure A show a reduction in plasma TG level for the glucose group?
The figure below summarizes the differences in LDL-C, non-HDL-C, apoB, and apoB/apoA-I.
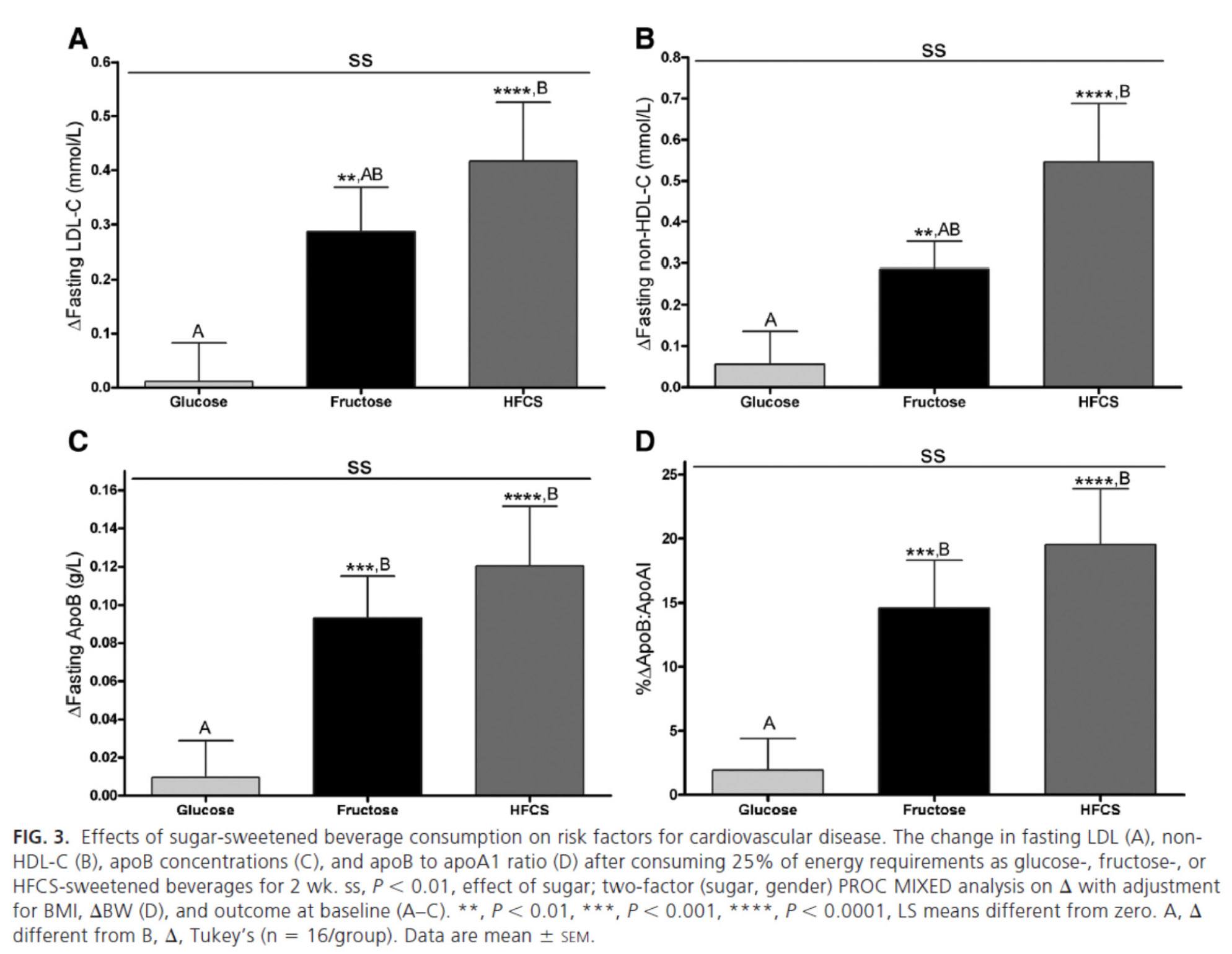
Again, the results were unmistakable with respect to the impact of fructose and HFCS on lipoproteins, and by extension, the relative lack of harm brought on by glucose in isolation. [Of course, removal of glucose and fructose/HFCS would have been a very interesting control group.]
One of the simultaneous strengths and weaknesses of this study was the heterogeneity of its subjects, who ranged in BMI from 18 to 35, in age from18 to 40, and in gender. While this provided at least one interesting example of age-related differences in carbohydrate metabolism (older subjects had a greater increase in triglycerides in response to glucose than younger subjects), it may have actually diluted the results. There were also significant differences between genders in the glucose group.
What was most interesting about this study was the clear difference between the 3 groups that was not solely a function of fructose load. In other words, the best outcome from a disease risk standpoint was in the glucose group, while the worst outcome was not in the all-fructose group, but in the 50/50 (technically 55/45) mixed group. This is a very powerful indication that while glucose and fructose alone can be deleterious in excess, their combination seems synergistically bad.
The role of saturated fat in cardiovascular disease
In the next week or two I’ll be posting an hour-long comprehensive lecture I gave at UCSD a few weeks ago on this exact topic. Rather than repeat any of it here, I’ll highlight one study that I did not include in that lecture. The study, Effect of a high saturated fat and no-starch diet on serum lipid subfractions in patients with documented atherosclerotic cardiovascular disease, published in 2003, treated 23 obese patients (average BMI 39) with known cardiovascular disease (status post coronary artery bypass surgery and/or stent placement) with a high-fat ketogenic diet. Because the study was free-living and relied on self-reporting, not all subjects had documented levels of elevated serum B-OHB. However, the subjects were instructed to avoid starch and consume 50% of their caloric intake via saturated fat, primarily in the form of red meat and cheese. There were no restrictions on fruits and vegetables, which may have accounted for the observation that not all subjects were ketotic during the 6-week intervention. In total, only 5 of the 23 patients achieved documented ketosis.
All of the subjects were on statins and entered the study at a goal LDL-C level target of 100 mg/dL, which may have been the only way the authors could get the IRB to approve such a study.
The table below shows the changes in lipoprotein fractions following the intervention (there was no control group):
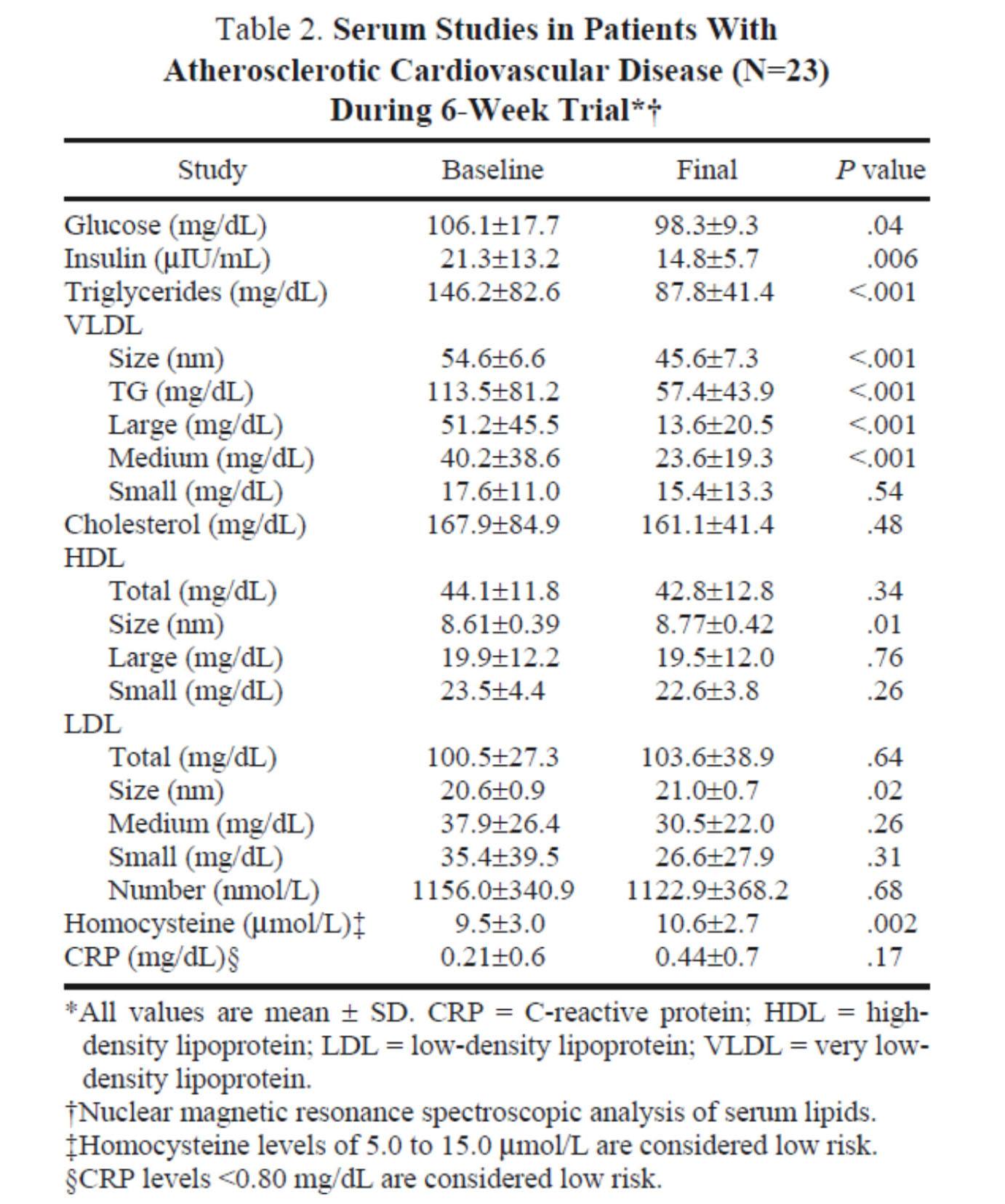
This study was conducted during the height of the “outcry” over the Atkins diet. While most doctors reluctantly agreed that Dr. Atkins’ diet could reduce body fat, most believed it was still very dangerous. In the words of Dean Ornish, “Sure you can lose weight on a low-carb diet, but you can also lose weight on heroin and no one would recommend that!”
Fair point. In fact, the authors of this study acknowledged that they “strongly expected” this dietary intervention to increase risk for cardiovascular disease, which is why they only included subjects on statins with low LDL-C. However, as you can see from the table above, the authors were startled by the results. The subjects experienced a significant reduction in plasma triglycerides and VLDL triglycerides, without an increase in LDL-C or LDL-P. In fact, LDL size and HDL size increased and VLDL size decreased – all signs of improved insulin resistance. Furthermore, fasting glucose and insulin levels also decreased significantly. The mean HOMA-IR was reduced from 5.6 to 3.6 (normal is 1.0) and TG/HDL-C from 3.3 to 2.0 (normal is considered below 3, but “ideal” is probably below 1.0) in just 6 weeks. Taken together, these changes, combined with the dramatic change in VLDL size, suggest insulin resistance was dramatically improved while consuming a diet of 50% saturated fat!
As all of these patients were taking statins, we’re really robbed of seeing the impact of this diet on LDL-P, which did not change. Also, CRP levels rose (though not clinically or statistically significantly).
Putting it all together
It is very difficult to make the case that when carbohydrates in general, and sugars in particular, are removed or greatly reduced in the diet, insulin resistance is not improved, even in the presence of high amounts of saturated fats. When insulin resistance improves (i.e., as we become more insulin sensitive), we are less likely to have the signs and symptoms of metabolic syndrome. As we meet fewer criteria of metabolic syndrome, our risk of not only heart disease, but also stroke, cancer, diabetes, and Alzheimer’s disease goes down.
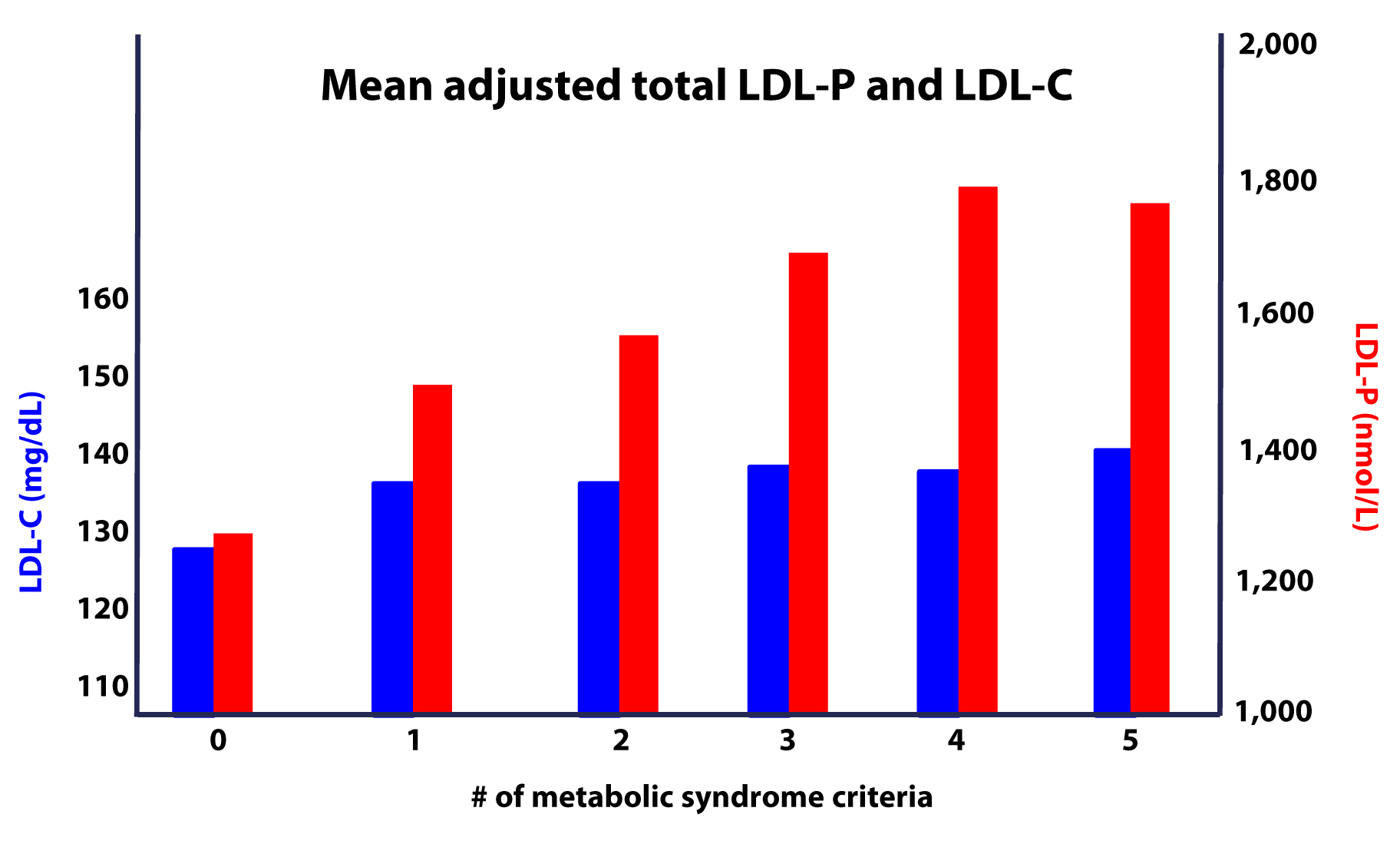
Furthermore, as this study on the Framingham cohort showed us, the more criteria you have along the spectrum of metabolic syndrome, the more difficult it becomes to predict your risk, due to a widening gap in discordant risk markers, as shown in this figure.
As I noted at the outset, the “dream” trial has not yet been done, though we (NuSI) plan to change that. Until then each of us has to make a decision several times every day about what we will and won’t put in our mouths. Much of this blog is dedicated to underscoring the impact of carbohydrate reduction on insulin resistance and metabolic syndrome.
The results of the trials to date, combined with a nuanced understanding of the lipoprotein physiology and their role on the atherosclerotic disease process, bring us to the following conclusions:
- The consumption of sugar (sucrose, high fructose corn syrup) increases plasma levels of triglycerides, VLDL and apoB, and reduces plasma levels of HDL-C and apoA-I.
- The removal of sugar reverses each of these.
- The consumption of fructose alone, though likely in dose-dependent fashion, has a similar, though perhaps less harmful, impact as that of fructose and glucose combined (i.e., sugar).
- The addition of fat, in the absence of sugar and starch, does not raise serum triglycerides or other biomarkers of cardiovascular disease.
- The higher the level of serum triglycerides, the greater the likelihood of discordance between LDL-C and LDL-P (and apoB).
- The greater the number (from 0 to 5) of inclusion criteria for metabolic syndrome, the greater the likelihood of discordance between LDL-C and LDL-P (and apoB).
I would like to address one additional topic in this series before wrapping it up – the role of pharmacologic intervention in the treatment and prevention of atherosclerotic disease, so please hold off on questions pertaining to this topic for now.



Have any of the cholesterol posts discussed the role of oxysterols in atherosclerosis/disease processes in general? I started keto with a normal BMI and a fairly exemplary basic lipid profile (HDL 106, LDL 54, TG 45), so am not overly concerned about CAD in particular. However, I am somewhat concerned that eliminating fruits/legumes/starchy veg while increasing my consumption of oxysterols (via dairy, cooked fatty meats, heated oils) could increase my net oxidative load, if you will.
I work in clinical research (specifically, oxidative stress and neuromuscular disease) and realize the data are quite murky regarding the risks/utility of antioxidant supplementation (especially vs. intake from food sources). However, I wonder if it may be warranted for those who have greatly reduced their intake of AOX-rich fruits with a concomitant increase in oxysterol intake.
My thinking is that AOX’s documented ability to inhibit PUFA oxidation might curtail the production of ROS/free radicals that could trigger cholesterol oxidation downstream. For those of us in a low risk category for CAD, carotenoids, tocopherols, mineral ascorbates, even flavanoids are at best theoretically beneficial, and at worst, benign.
I’d like to hear your thoughts on the matter!
An article on the biological role of oxidized LDL, for anyone interested: https://www.jbc.org/content/272/34/20963.full
Nico, I’ve discussed it in various comment threads, but not yet in posts. Part of the problem is we don’t have a really good way to measure oxLDL. LpPLA2 is probably the best, and I’m still not sure how good it is. Clearly oxidation plays a role in atherosclerosis, but it’s not entirely clear (to me, at least), how to quantify it.
Peter, If I had been that girlfriend in the parking lot I would have asked you to explain the significance of the area under the curve so that I could grasp exactly what you’re talking about and we probably would have been there for several hours hashing through it all! Thank goodness for people like you to share your enthusiasm and quest for understanding in so many areas important to us all. That energy, enthusiasm and thirst for knowledge comes through your on-line writings and talks. It is a real gift to all of us out here in the world trying to learn, understand and do the right thing by ourselves and our communities.
Thank you!
Funny. I’m sure she has long forgotten that day…though probably has an awful memory of some loser she once dated.
I wonder how smoking and then quitting smoking affects cholesterol, if any?
The link “To see an important reference on this topic, please look here.” is not working…for item #7.
7. Eating cholesterol has very little impact on the cholesterol levels in your body. This is a fact, not my opinion. Anyone who tells you different is, at best, ignorant of this topic. At worst, they are a deliberate charlatan. Years ago the Canadian Guidelines removed the limitation of dietary cholesterol. The rest of the world, especially the United States, needs to catch up. To see an important reference on this topic, please look here.
Thanks Peter, obviously you share priceless information.
My question is, can you over ingest butter and other fats, ala bulletproof coffee for example, way above what your body needs and can handle and therefore have your cholesterol go thru the roof and liver enzymes elevated, whereas, if you eat 2 to 3 meals a day and use fat as a flavor like ingredients (cook in butter for example) cholesterol and liver enzymes would be normal, in other words is there a point in the curve that a ketosis diet can go from producing positive health results to negative ones. I assume every dirt would have such a point but is it easier to reach that point if you add fat to drinks, coffee etc..
Thanks in advance
Absolutely, and it varies by person.
That means dietary fat DOES affect cholesterol levels, may be indirectly? With the aim of losing weight when one is fat adapted and they also are on a high fat diet, could there be too much fat available for their body in this situation? Could this explain why some people experience very high ldl on a low carb diet?
In some cases, yes. Tom Dayspring addressing this in a LecturePad post. I’ll revisit at some point.
Thanks Peter. Would really appreciate title/link of Dr Tom Dayspring’s post if it is out.
Lipidaholics case #291
Thanks Peter, helpful as ever. It is a great post and I would recommend reading of it to all who have experienced high LDL-C and LDL-P on a low carb diet. I truly hope that we do not go from one extreme to the other: from demonisation of fat to over consuming it believing that cholesterol/LDL-P is not relevant and it is all about inflammation as that’s what is being proposed by many in low carb community.
I enjoy very much of your posts, and please help me to understand the mechanism of atherosclerosis.
The key of atherosclerosis is to understand how LDL particles go through single-layer vascular endothelial cells of arteries into the sub-endothelium. I have the following three related questions:
(1) Can the LDL particles enter the single layer endothelial cells by transcytosis: endocytosis first, and then exocytosis? If yes, please cite the literatures;
(2) If the number of LDL particles is major contribution factor to atherosclerosis, then (a) will the diameter of artery affect the atherosclerosis process; (b) why it occurs mainly at coronary artery or carotid artery, and rarely at the other arteries?;
(3) Does calcium play any roles in the atherosclerosis process? If yes, could you comment its role?
(1) not to my knowledge
(2) great question, and the issue is probably multifactorial — a combination of artery diameter (i.e., more susceptible to narrowing) and local factors that may damage the intima. Many arteries in the body experience athero, including large ones (aorta, femoral, renal). Lastly, collarerization plays a role in mitigation outside of the CV system
(3) yes, addressed in previous posts
Thank you for your prompt response to my questions.
(1) Without understanding the mechanism of LDL being transported from the lumen side to the other side of sub-endothelium, all of the theories are only hypotheses to me. We need to understand the true mechanism to determine the real root cause of atherosclerosis.
The endocytosis is proven by Brown-Goldstein when the cells themselves decide that they need more cholesterol by synthesizing more LDL receptors. If they don’t need more cholesterol, then they reduce or stop synthesizing the receptor. Thus, the cells are not forced to synthesize more receptors to take cholesterol disregarding that there too many LDL particles in the lumen. In my opinion, it is very unlikely that endothelial cells would import LDL particles by endocytosis and export by exocytosis to the other side of sub-endothelium simply because there are too may LDL particles in the lumen.
Is it possible that there exist gaps or cracks in the endothelium cells; or the cells are forced to endocytosize not normal cholesterol but the oxidized cholesterol thru different receptors as suggested by some literatures; or from damaged endothelial cells; or too much calcium; or etc.?
(2) If the number of LDL particles, LDL-P rather than LDL-C, has stronger impact in atherosclerosis, then it indicates that the number of times that a cell contacting with LDL particle in a unit of time is a more important determinable factor than the number or the size of particle. If this is case, then we should consider the velocity in addition to the number of the particle. The atherosclerosis should occur more frequently at the location where the number of contact time is higher.
Can you comment on the role of diet in patients with Apo E 3/4 or 4 genotype? The conventional wisdom is that these patients should go on a low fat diet to improve cholesterol levels. Is there a decreased cardiovascular risk in these patients if their LDL cholesterol decreases on a low fat diet? What diet advice would you give to a ApoE 3/4 patient? Thank you!
Complex. 3/4 and 4/4 patients require special care and lots of tweaking to get right. Usually tolerate SFA less well and need less fat in diet. Also, rarely respond to monotherapy for lipid lowering.
Is it a good idea to have Apo E genotype tested for people who have experienced rapid increase in their LDL-C/LDL-P on a LCHF diet?
I think so, but that may not account for the response. ApoE is one of the few genes I think it’s worth knowing early in life.
> ApoE is one of the few genes I think it’s worth knowing early in life.
When you get a chance, it might be useful to list the diet-related genes worth knowing about. If possible, please also identify some labs that report them. What actual adjustments to make for each might be beyond the scope of such an article.
The only genes I’ve stumbled upon so far are AMY1, ApoE and MTHFR – and the low-cost DIY lab 23andme apparently does not report AMY1.
I suspect that although the keto-paleosphere is distracted at the moment by resistant starches (and possibly “safe starches”) and is about to confront gut biome, the next big thing will be genotype-specific diets, and that’s going to require knowing.
Thanks Peter, can E3/4, 4/4 still get away with a high fat non ketotic diet by tweaking with SFAs and PUFAs or is it omega 6 that they need to be careful about? Would you please recommend any good readings (not too technical) on Apo E4 in the context of how such genotypes respond to dietary fat. Many thanks
Hi Peter, have you heard of Stephanie Seneff of MIT and this article: APOE-4: The Clue to Why Low Fat Diet and Statins may Cause Alzheimer’s. She discusses the defect as a cholesterol transport problem: “To summarize, I hypothesize that, for the apoE-4 Alzheimer’s patients, defective apoE has led to an impaired ability to transport fats and cholesterol from the blood stream, via the astrocytes, into the cerebrospinal fluid. The associated high blood serum cholesterol is an attempt to partially correct for this defect.”
Dr. Perlmutter speaks of the need for low carb with increased healthy saturated fats to protect the brain even in cases of ApoE 4. As far as I know, there isn’t anything definitive suggesting to lower fat for 3/4, 4/4. Wouldn’t it put brain health at risk to do so? Thank you, Maryann
It’s not clear that optimizing around brain health and heart health have the same path in apoE 4/4 and 3/4.
Thank you Dr. Attia, that’s interesting. A person would have to choose which he prefered to treat? Heart health or brain health and possibly to the detriment of the other?
It’s a bit more nuanced than that.
I would roughly guess that non ketotic low carb and low SATURATED fat might be the way to go for E3/4, 4/4 people?
I am 68 and my cholesterol is 304. HDL 66, Trig. 98, LDL 218. The cholesterol went up from 250 to 304 after gallbladder removal. I have lone afib also but controlled with mag, etc. My cardiologist wants me to go on statins. I have no other heart issues, CT scan showed zero plaque and no risk of heart disease. The doctor was stunned as he thought I would have plaque. I think my cholesterol is familial. I did have the VAP test once which showed about 50/50 fractionated Ldl. A few of the numbers were off on the bad side. My Lpa is within range. My Ldl’s are always high. So, I’m wondering if I should do anything based on my results or? I do exercise and eat fairly low carb. Strictly no processed foods, grass fed beef, etc. Thanks for your info even though I don’t understand all of the in depth cholesterol info. It’s good to read as most docs just go by simple numbers and prescribe pills. I always said that it’s way more complicated than that and most docs are not treating people individually.
Hi Dr. Attia
I know this has only a vague connection to this post, but I am looking to know what are the timescales for enzymatic changes to the liver. For example, if I start eating lots of cholesterol (by the spoon fulls…), how much time will it take to my liver to downplay the cholesterol synthesis pathways? And if after that time I stop all cholesterol consumption – how much time until the liver starts cholesterol production again?
Thanks.
I don’t know exactly, but I’ve seen the changes in as little as 4 weeks.
Dr. Attria,
I am looking forward to your article on APOE 3/4, 4/4. In the meantime I have a quick logical question, if one could get their gut in working order, reduce inflammation, eat a healthy diet, wouldn’t the whole APOE questions be reduced or eliminated? Since APOE 4 is the ancestral gene and our ancestors ate organ meats preferentially which is loaded with saturated fat, couldn’t one draw the conclusion that it is not the saturated fat per se but what is also in the diet that effects saturated fat metabolism?
I don’t really know. It’s unlikely the apoE 4 variant would have survived so long if it did not confer some benefit early in life (i.e., pre-reproduction), but that says nothing about the risks it poses later in life.
What could cause discordance between LDL-P and LDL-C in an individual with low triglycerides? Does LDL carry stuff other than cholesterol and triglycerides (vitamins and antioxidants?) I have been taking 4000 IU of Vitamin D as well as a number of anti-oxidants, and have seen a jump in D levels from 12 to 36. I had discordance in my first NMR and have been on a LCHF diet for maybe 6 months. Additionally, is there some sort of a half life for LDL? Typically does reduction in particle number happen steadily over a long period of time?
Thanks so much for your work at NUSI and on this blog.
Particle size, phospholipids, a few other factors. Half life for LDL particle depends on size: for larger particles (pattern A), about 36 to 48 hours; for smaller particles (pattern B), more than 72 hours.
hello Peter,
I am pursuing the question whether I can safely change my medication now that I have changed my diet to vey low carbs.
I do still have two signs of metabollic syndrome: treated for blood pressure and persistent, but not worsening, HbA1c at 5.9
TG is 60 and HDL is 60. waist is small enough. Doctor detected low visceral fat on physical exam.
I have succeeded in getting an order for advance lipoprotein testing, so I now need to know:
1. how long to go off of atorvastIn 40mg before I can get a valid baseline LDL-P.
2. How safe is it to go off the statin?
If the results are favorable, than I would also need to know
3. which other lab measures (inflammatory markers?) would be needed, if any, for a statin decision,and
4. which RCTs on post- menopausal women, or good meta analyses of them, would support changing the stain recommendation?
Can you help me find answers to these questions?
I am not asking for advice about treatment, just information from your data bank to help me navigate the system and communicate with my doctors.
Thank you so much for your hard work for NUSI, for your advocacy and TED Talk, and for this educational blog.
Hi Peter,
I was reading the abstract of a study that said that when compared with a VLCHF diet, “an HCLF diet had more favorable effects on the blood lipid profile. This suggests that the potential long-term effects of the VLCHF diet for CVD risk remain a concern”. (https://www.ncbi.nlm.nih.gov/pubmed/18174038)
But reading the full article, the conclusions say that “The HCLF diet had more favorable effects on LDL-C, whereas the VLCHF diet had more favorable effects on TG and HDL-C, suggesting that the latter approach may have relevance for the management of the metabolic syndrome.” (https://www.sciencedirect.com/science/article/pii/S0735109707032597)
Am I the only one that sees a Jakyll/Hyde transformation here? I thought pubmed was a reliable source.
PD: in Table 3 a change in LDL-C from 3.24 to 3.19 is NOT an increase of 0.06.
I have a correction to make: the transformation in the conclusions is not pubmed’s fault: the “hyde-conclusions” can be found at the beginning of the full article.
Sorry pubmed. My wrong.