A couple of weeks ago Tim Ferriss and I were having dinner and the topic of cancer came up. As some of you may know my background is in oncology, specifically in exploring immune-based therapies for cancer by exploiting the properties of regulator T-cells. But that was a long time ago. Like many of you, I expect, I’ve also been personally impacted by cancer having lost a friend to glioblastoma multiforme (GBM). I often describe GBM to people as one of the cancers that gives cancer a bad name. When I went to medical school I planned on becoming a pediatric oncologist, and though I ultimately chose to pursue surgical oncology, my interest in helping people with cancer never wavered.
Over dinner that night, Tim asked me if I could write – in about 1,000 words! – a post on cancer that would be interesting and digestible to a broad audience. “1,000 words?!,” I asked. “How about 30,000 words?,” I responded only half kidding. After explaining why I couldn’t possibly write such an abridged version, Tim talked me into it. And so, I plan to accept the challenge and hope to provide readers with such a post (it will be on Tim’s blog when I do so), hopefully in the next month or two.
For an introduction, however, I’d like take a step back and place this topic in a broader context. I don’t need to say much about cancer that you don’t already know. You probably know that about one in three Americans will develop cancer in their lifetime, and you probably know that about half of them will succumb to the disease. What you may not know, however, is that we have made virtually no progress in extending survival for patients with metastatic solid organ tumors since the “War on Cancer” was declared over 40 years ago. In other words, when a solid organ tumor (e.g., breast, colon, pancreatic) spreads to distant sites, the likelihood of surviving today is about what it was 40 years ago with rare exceptions. We may extend survival by a few months, but not long-term (i.e., overall) survival.
We screen better today for sure, but subtracting lead-time bias, it’s not clear this extends overall survival. We’ve had success in treating and even curing hematologic cancers (e.g., some forms of leukemia and lymphoma). Certainly testicular cancer patients (especially seminomatous) are better off today and those with GI stromal tumors (GIST), too. Surgical control of cancer is much better today and some local treatments (e.g., specific radiation), too. But for the most part, when a patient has metastatic cancer today, the likelihood of living 10 more years is virtually unchanged from 40 years ago.
About a year ago, I was asked to give a talk about metabolic disease to a group of physicians. But before I spoke, a very astute and soft-spoken oncologist, Dr. Gary Abrass, gave the following introduction as a way to frame the context of my talk. After all, I’m sure many in the audience were wondering what could a discussion of insulin resistance have to do with cancer. I have thought often of his words that night in the many months since he so eloquently and informally introduced me.
I asked Dr. Abrass if I could have a copy of his talk and share it with you, to which he kindly agreed. Below is, nearly verbatim, the talk he delivered that night. (Dr. Abrass did give me the liberty of tweaking the text a bit, for emphasis and clarity.)
How have we fared in the War on Cancer?
On December 23, 1971, President Nixon declared war on cancer by signing the National Cancer Act. I was going to title this, “40 years in 4 minutes,” but I think this will take me a bit longer. At the time I was a third year medical student. Two years before, Neil Armstrong had inflated our national pride by setting foot on the moon, and there seemed no scientific goal unachievable. Activist Mary Lasker published a full-page advertisement in The New York Times: “Mr. Nixon: You Can Cure Cancer.” And she went on to quote Dr. Sidney Farber, Past President of the American Cancer Society and whose name now sits atop the Harvard Cancer Center, “We are so close to a cure for cancer. We lack only the will and the kind of money and comprehensive planning that went into putting a man on the moon.” Since then, the federal government has spent well over $105 billion on the effort.
Forty years later, Dr. Farber’s prophecy remains unfulfilled. In 2012 cancer killed an estimated 577,190 people in the United States. The death rate, adjusted for the size and age of the population, has decreased by only 5 percent since 1950. And most of this decline is due to mammography screening in breast cancer and cessation of smoking, resulting in less lung cancer in men.
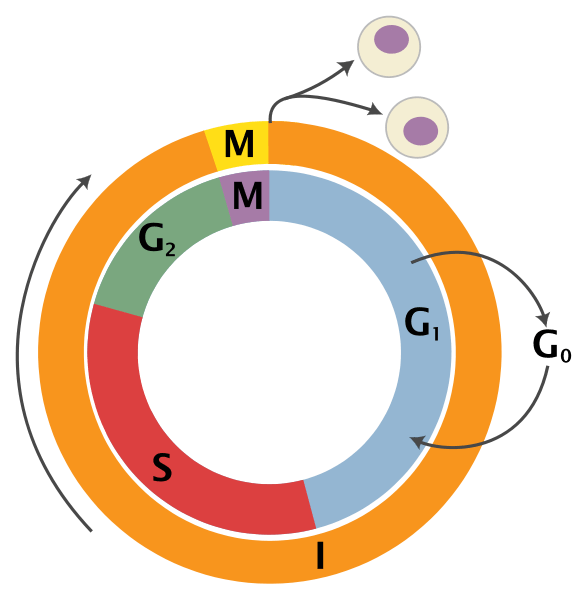
We have however developed a greater understanding of the biological and molecular basis of cancer. When I was a medical student, this graphic summarized what we knew about the growth cycle of the cancer cell.
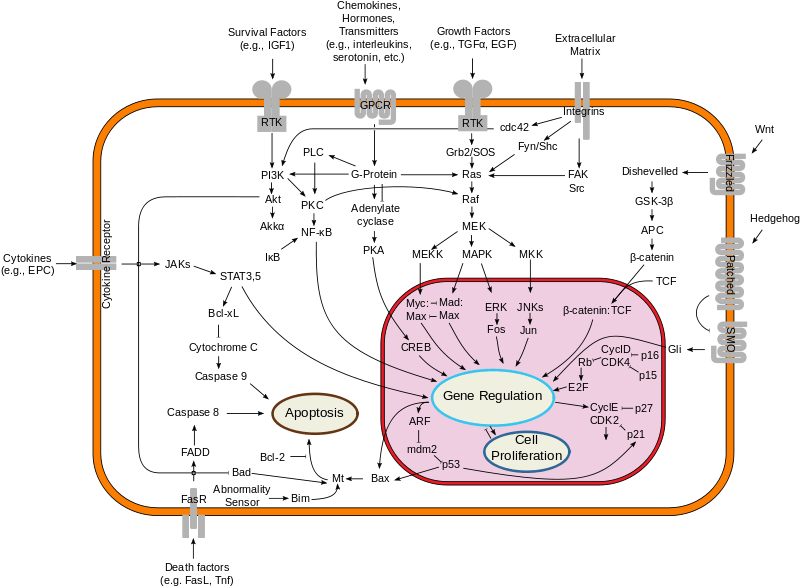
The next graphic demonstrates what we have learned, a truly overwhelming accomplishment, a dizzying array of interconnecting signaling “pathways,” and spawned a whole new field, “translational medicine.”
With the completion of the Human Genome Project, new sequencing technologies have also opened up the prospect of personal genome sequencing as an important diagnostic tool. A major step toward that goal was the completion of the sequencing of the full genome, first on James D. Watson, one of the co-discoverers of the structure of DNA. In fact, Steve Jobs had personal genome sequencing. The price is down to $1,000 and Mayo Clinic Proceedings recently had an article raising the concern of direct to consumer advertising for genomic sequencing. We are now able to sequence gene by gene, pathway by pathway, the genetic code of some cancers. New “smart” drugs have been developed that target various mutations in these pathways. And currently there are over 800 “targeted agents” in clinical development. These drugs have been described by some investigators as “The Holy Grail,” but the clinical results suggest more of a commemorative cup for a “Happy Meal.”
While there is nothing unique about this paper, it is a good example of a typical negative trial targeting the IGF-1 receptor with a monoclonal antibody. The lack of response does not make us question the role of the IGF pathway as a prime driver of malignancy, but rather demonstrates the ability of the cancer to resist therapy. It seems there is so much redundancy in the system that the cancer finds alternate pathways. The cancer cell continues to defend itself, to bob-and-weave like the arcade game “Whack-a-Mole.”
So the investigators combined the IGF-1 monoclonal antibody with various other therapies: standard platinum based chemotherapy regimens, the “small molecule” tyrosine kinase inhibitor gefitinib (Iressa) targeting another common pathway (EGFR), and also the mTOR inhibitor temsirolimus (Torisel) which targets the IGF-1 pathway a bit further along. In any case, the point is, it still didn’t work. But why?
Investigators have mapped the genome of a typical lung cancer patient and found over 50,000 mutations. That’s a lot of targets! Granted they are not all ‘driver’ mutations, some are ‘passenger’ mutations. The patient whose genome was mapped was a 51-year-old man who’d reported smoking 25 cigarettes a day for 15 years. At 50,000 mutations, it works out to one mutation for every 2.7 cigarettes.
Last year the New England Journal of Medicine published a study by Gerlinger and colleagues. These investigators looked at intra-tumor heterogeneity. They performed a molecular dissection. They found diversity within the tumor itself. Each cancer is not cancer but, indeed, it is CANCERS. In other words “all cancers are rare cancers.” Let me repeat this point. Each tumor is a collection of heterogeneous – not homogenous – mutated cells. This article has engendered some discussion and some controversy. Many say that the results of the study bring the idea of personalized medicine to a halt, or at least dramatically slow it down. Are there exceptions? Yes, but they are rare. One example of an exception to this revelation is chronic myelogenous leukemia (CML), a cancer with a known single driver mutation.
In September 2011, biologist Dr. Alasdair MacKenzie of the University of Aberdeen, speaking at the British Science Festival in Bradford, explained that researchers trying to fully understand how our DNA causes disease might not be looking in all the right parts of the genome. The past decade of genetic studies has revealed that our 3.2-billion-long-DNA-letter code is more complex than anyone could have thought. More than 98% of the human genome does not encode protein sequences. It’s been referred to as “Junk DNA” and thought not to have a function, but maybe more correctly is that we do not know the function. He refers to this as the “dark matter” of the genome. And it’s thought that some of these “alternate pathways”, in which our resourceful cancer cell seeks refuge, may reside here. If this was not complicated enough, the new field of Epigenetics has grown exponentially resulting in a widening of the battlefield.
These are factors that can affect the expression of genes without causing mutations, turning switches on and off. In terms of cancer, much of this research has concentrated on what are called “Nononcogenic stress targets.” We can stress an organism in many ways: heat, poison, starvation, suffocation or more scientifically thermal, chemical, metabolic and oxidative stress. Organisms have an ingenious way of responding to such stress. In the 1960s an assistant in FM Ritossa’s lab accidentally boosted the incubation temperature of Drosophila (fruit flies), and when later examining the chromosomes, Ritossa found a “puffing pattern” that indicated the elevated gene transcription of an unknown protein.
This was later described as the “Heat Shock Response” and the proteins were termed the “Heat Shock Proteins.” This same HSP increases survival under a great many pathophysiological conditions. The HSP70s are an important part of the cell’s machinery for protein folding and help to protect cells from stress. While it enhances the organism’s survival and longevity under most circumstances, HSF1 has the opposite effect in supporting the lethal phenomenon of cancer. These proteins enhance the growth of cancer cells and protect tumors from treatments. This remarkable protein affords a protective response to other proteins in the cell, acting as a “chaperone” preventing them from mis-folding or “denaturing,” like when a boiled egg white turns opaque. These heat shock proteins are expressed at high levels in many tumor types: breast, endometrial, lung, prostate, even brain tumors. HSP overexpression signals a poor prognosis in terms of survival and response to therapy. HSP’s are now on the radar as a key target in the ongoing battle. This protein folding stress response is a hot topic in current cancer research. I have been communicating with Dr. Debu Tripathy who is currently studying epigenetic changes and protein folding stress responses associated with obesity. This protein folding stress response affords the cancer cell a survival advantage, and we share this protective mechanism with a fly, such a distant relative in our family tree, that one can only conclude that the cancer cell has hijacked this maneuver, this protective drive for immortality from the legacy of 100’s of millions of years of evolution…such a resourceful and formidable opponent.
In any case, when the Human Genome Project was near completion, President Clinton hosted a White House ceremony and announced that, “it will revolutionize the diagnosis, prevention and treatment of most, if not all, human diseases, and that humankind is on the verge of gaining immense new power to heal.”
The hubris of it all. It’s reminiscent of the quote of Sidney Farber. Hopefully this is not tempting fate. Theologians tell us the only unforgivable sin is pride. The increasing complexity of the science is affording us quite a dose of humility. British Physicist Brian Cox said that “being at the junction of the known and the unknown is a beautiful place to be for a scientist,” but it seems the more we know, the more we don’t know. Not unlike Winston Churchill’s characterization of Russia as “a riddle wrapped in a mystery inside an enigma.” Not unlike modern theoretical physics, one questions whether we are capable of understanding the complexity of the science. Hopefully it’s not like trying to teach my dog quantum theory. We are so smart, but it seems that the cancer cell is smarter. It bobs and weaves, slips our punches, and when we back it into a corner, it defends itself in remarkable ways borne of millions of years of evolutionary acumen much of it hidden in the dark matter of our genome.
Maybe we should call a truce in the War on Cancer and concentrate on prevention. Besides smoking, the most preventable cause of cancer seems to be obesity. It is generally thought that obesity may account for about a third of many cancer types, particularly breast, colon, uterus, kidney and esophagus. Obesity is a risk factor for type II diabetes and these patients are not only more likely to get cancer, but to have poor outcomes. Other speakers will explore the relationship of obesity and cancer, the epidemiology and the science, and see if this lends support to any practical prevention measures.
Gary Abrass, M.D.
April 19, 2012
Afterword
Just as the best way to get in shape is not to ever get out of shape, the best treatment for cancer is almost assuredly not to get cancer. And that’s clearly the theme of the introduction Dr. Abrass gave me. But I’m sure many of you are asking a more important question — what happens if I or someone I care about has cancer? If you can be patient with me, I do plan to address this, to the best of my understanding, in the coming months.