Age-related hearing loss (HL) is often represented in popular media as little more than a source of comic relief – a misheard statement leads to a hilarious non-sequitur when a doddering elderly character attempts to respond. But hearing loss is no laughing matter. Apart from affecting quality of life and ability to live independently, HL has been associated with dementia and has been proposed as a potential biomarker – or even causative factor – for cognitive decline.
As I discussed in a recent “Ask Me Anything” episode, evidence for this relationship has come from a number of epidemiological studies, which have established clear correlation but cannot provide insights regarding causation. But last week, investigators Lin et al. published results of their ACHIEVE randomized trial, which sought to find this missing puzzle piece by investigating whether interventions aimed at treating hearing loss could slow or prevent cognitive decline. So what did we already know about the relationship between hearing loss and dementia, and how do the results from ACHIEVE add to this knowledge?
How aging affects hearing
HL is extremely common in older adults, with over 50% of American adults aged 70+ suffering from some level of hearing impairment. Moderate or greater HL affects 13.4 million adults aged 60 or older, and bilateral age-related hearing loss is the most common form. There is a lack of awareness about the sheer numbers of people affected by hearing loss. This is a topic I wrote about in a previous newsletter, along with recommendations to prevent hearing loss. (Since writing that piece I’ve become obsessed with protecting my hearing at all costs and have invested heavily in hearing protection for all activities that expose me to meaningful noise.)
Hearing requires the sensory auditory system and processing of auditory signals by several parts of the brain; both of which often diminish with age. The ability to detect sound diminishes with age due to degeneration and atrophy of hair cells and structures in the inner ear. Neurons that transmit auditory input from the ear to the brain also atrophy with age, resulting in hearing loss and decreased ability to discriminate speech.
In addition to perceiving sounds, many parts of the brain are needed to process auditory information to produce comprehension. Here too, age-related degeneration can result in hearing loss. Studies suggest that processing deficits in the brainstem account for difficulties in understanding speech independent from the ability to hear tones in audiometric testing. Changes in brain morphology have been observed in adults with HL as well as changes in cortical networks needed for speech perception. Thus, changes in both the ear and the brain can affect hearing.
Associations Between Hearing Loss and Dementia
Several observational studies have reported associations between HL and dementia. Analysis of participants in the Baltimore Longitudinal Study of Aging, for instance, found that the risk of dementia increased with the severity of HL. Among over 600 patients followed for a median of 11.9 years, the risk of developing dementia was significantly increased in those with mild hearing loss and increased in a log-linear fashion with HL severity, up to a hazard ratio of 4.94 (95% CI: 1.09-22.4) for severe HL (defined as a hearing threshold of >70 dB). That is a very big hazard ratio.
Similarly, a systematic review and meta-analysis of 36 studies (for a total of over 20,000 participants) found that age-related hearing loss was significantly associated with cognitive decline and dementia. The authors report an odds ratio (OR) of 2.00 (95% CI: 1.39-2.89) for the association between HL and cognitive decline and an OR of 2.42 (95% CI: 1.24-4.72) for the association between HL and dementia. These data indicate clear correlations, suggesting that age-related hearing loss may be a possible biomarker for cognitive decline and dementia. But the important question is that of HL directly contributing to dementia, thereby representing a modifiable risk factor, versus being either a marker of some other cause or itself a consequence of dementia.
Hearing loss as a risk factor for dementia
When the Lancet Commission released its 2020 report outlining 12 modifiable risk factors for reducing risk of dementia, HL figured prominently as one of the most critical variables, with a reported relative risk of 1.9 (95% CI: 1.4-2.7).
A JAMA study published in July of 2022 examined the proportion of dementia cases in the U.S. associated with the risk factors listed by the Lancet Commission. Their findings, presented in the table below, indicate a similar relative risk for HL but also a strikingly high communality – a calculated estimate of the extent to which a given variable correlates with others. A maximum score of 100% would mean that an apparent association between that variable and the outcome of interest (i.e., dementia) might be explained completely by other covariates, while a minimum score of 0% would mean that other covariates can account for none of the association. While HL tops the list in terms of relative risk, it’s important to note that over 80% of that risk may be attributable to other factors. Still, these models – which are based on epidemiological data – indicate that at least some of the association is due to HL itself. But why? How might hearing loss impact cognition?
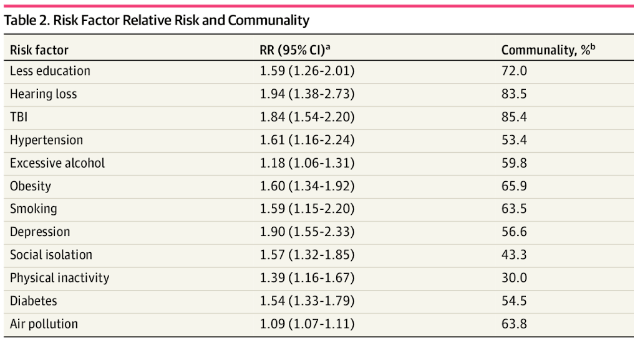
Table 1. Relative risk (RR) for risk factors for dementia [JAMA 2022]
Mechanisms hypothesized to explain the association between HL and dementia
One of the most common mechanisms hypothesized to explain the link between HL and dementia is that increased cognitive load brought on by HL promotes cognitive decline and dementia (reviewed in 2019). People with HL require great attention and concentration to understand speech. This may divert cognitive resources from other tasks and drain working memory, thereby contributing to cognitive decline. Further, this diversion of resources to hearing is thought to cause structural changes in the brain which contribute to cognitive decline and neurodegeneration.
A second hypothesis is the cascade hypothesis, which contends that a loss of sensory input leads to brain atrophy. This use-it-or-lose-it theory relies on experience-dependent neuroplasticity – the ability of the brain to change in response to environmental stimuli, and suggests that neuroplastic changes in the prefrontal cortex (rather than decreased cognitive reserve) is a mechanism linking HL to dementia. The loss of auditory input and use of neural pathways for processing auditory signals may lead to brain atrophy through disuse much as muscle atrophy is a consequence of disuse during bedrest. Consistent with this hypothesis, the temporal lobe – the area of the brain that processes auditory inputs – is also the first area impacted by Alzheimer’s disease. Further, it is possible that HL exacerbates brain pathology from amyloid-beta accumulation, neurofibrillary tangles, microvascular disease, etc.
If the cognitive load hypothesis or cascade hypothesis are true, then treating HL would presumably reduce risk of cognitive decline and dementia. But a third hypothesis holds that a common neurodegenerative process in the aging brain results in both HL and cognitive impairment. If this is true, then treating HL would not impact the risk of dementia, though HL may still be an early biomarker for dementia.
Can treating hearing loss delay dementia?
Before the ACHIEVE trial, clues into the benefits of hearing restoration on dementia risk came primarily from observational studies. For instance, a recent study of people with HL found that hearing aid users were less likely to develop mild cognitive impairment (MCI) than those who were hearing impaired but did not use hearing aids (HR=0.47; 95% CI: 0.29-0.74, P=0.004). Further, they found no difference in risk of MCI in individuals with normal hearing and those who had HL and used hearing aids to restore auditory function to normal levels. This latter point is significant, as it shows that the development of hearing loss is not necessarily accompanied or followed by MCI, which in turn suggests that reverse causation – i.e., that hearing loss is caused by incipient cognitive impairment – is unlikely to explain the association between the two.
However, observational studies are limited. Many rely on self-reporting of HL and hearing aid use instead of objective measures such as audiotone testing. Additionally, results of epidemiological studies may be confounded in that hearing aid users tend to be healthier and have a higher socio-economic status than those with hearing impairment who do not use hearing aids. To truly determine whether treatment of hearing loss might be an effective strategy for reducing dementia risk, we need data from randomized prospective controlled trials. This was the motivation for Lin et al.’s ACHIEVE trial.
The ACHIEVE trial
ACHIEVE was a randomized trial designed to test whether interventions to improve hearing could reduce risk of cognitive decline in those with age-related HL. The investigators recruited cognitively healthy adults aged 70-84 with bilateral HL from two populations: 1) individuals from the pool of participants in the ongoing Atherosclerosis Risk in Communities (ARIC) study, and 2) individuals recruited de novo for ACHIEVE. Collectively, these participants were randomized to either a hearing intervention group (n=490) or a health education control group (n=487). The hearing group received hearing aids and supplementary hearing-assistive technologies and met with an audiologist every 1-3 weeks, while controls met with health educators every 1-3 weeks for personalized, interactive education programming in prevention of chronic disease and disability. Changes in cognition were determined using a battery of standardized cognitive tests performed at six-month intervals for three years following randomization.
By the end of the three-year follow-up period, the intervention and control groups were indistinguishable in global cognition scores. Secondary analyses for executive function, language, and memory tasks also showed no significant differences between groups. However, when the authors conducted pre-specified subgroup analyses for participants from the ARIC study versus participants recruited de novo, they found that the hearing intervention resulted in significantly smaller reductions from baseline in global cognition and language scores than the health education control intervention, meaning that the hearing intervention group showed less cognitive decline. No significant differences in cognitive outcomes were observed between interventions for the de novo cohort, who notably had fewer risk factors for cognitive decline and higher cognitive scores at baseline than the ARIC participants.
How should we interpret these findings?
The lack of between-group differences in cognitive trajectories in full-cohort analyses raises doubts about whether hearing loss may contribute to cognitive decline – and whether hearing loss treatment can therefore slow or prevent cognitive decline – but these results are far from conclusive. This trial is not without its share of limitations, some of which might have increased the likelihood of obtaining negative results even if a real causal relationship exists.
Though the study cohort was relatively small, the fact that intervention and control groups were virtually identical in pooled analyses of cognition changes suggests that insufficient statistical power is not to blame for the apparent lack of effect. However, the cognitive benefit of hearing interventions observed in the ARIC subgroup hints that the duration of the study may have been too short.
The ARIC subgroup started the study at higher risk of cognitive decline; they were, on average, older and more likely to be female, and they had less education, higher rates of hypertension and diabetes, and lower baseline cognitive scores than the de novo subgroup. In other words, the ARIC participants may have represented a group that was moving down the path of cognitive decline faster than their counterparts, allowing meaningful separation between intervention groups within the three-year study period, whereas the more slowly declining de novo cohort may have needed a period longer than three years to see significant benefits from hearing interventions. The inconsistency between sub-groups may therefore be analogous to the inconsistency observed in statin trials for secondary prevention (i.e., in patients with established cardiovascular disease at baseline) and trials for primary prevention (i.e., in patients who are healthy at baseline). Of note, the de novo cohort accounted for roughly three quarters of all study participants, so for pooled analyses, a lack of apparent effect in this group would have overwhelmed any benefit observed in the ARIC cohort.
Additionally, the success of hearing interventions in restoring normal hearing was evaluated based on participants’ self-perception rather than on objective hearing tests. Because the participants could not be blinded to the intervention they received, these self-reports could have been subject to a placebo effect, in which patients believed they were hearing better simply because they were wearing hearing aids.
Conversely, the appearance of a benefit from hearing interventions in the ARIC cohort might have been caused by another source of potential bias – the cognitive tests, some of which relied on auditory stimuli. As earlier studies have noted, those with worse hearing may be less capable of hearing auditory tests or its instructions, resulting in poorer cognitive performance despite normal cognitive ability.
In all, these limitations mean that results from this trial should be viewed as an important starting point rather than as a definitive answer to the question of causality in HL and dementia.
Don’t ignore hearing loss
Age-related hearing loss is a largely treatable condition that affects millions of elderly Americans. Many observational studies have provided firm evidence that HL is correlated with cognitive decline, but even now, with the publication of the first randomized trial on hearing interventions and cognition, we can’t make any definite conclusions about a causative role for HL in increasing risk of dementia or cognitive impairment.
Yet even without a clear answer on this question, we still know that screening for and treating hearing loss is a relatively easy intervention that improves quality of life and possibly delays the onset of or reduces the risk of dementia. When it comes to extending lifespan, other ongoing studies may eventually shed additional light on whether hearing loss contributes to the deadly affliction of dementia. But when it comes to improving healthspan and quality of life, this is a no-brainer. Patients with cognitive impairment should be evaluated for hearing loss, and patients with hearing loss should not ignore it and should aim to restore this sensory modality as best they can.
For a list of all previous weekly emails, click here.



