On the subject of nutrition, we devote most of our attention to the macro: energy balance and the distribution of carbohydrates, proteins, and fats. But we mustn’t ignore the micro: the finer points of the topic – including sufficient consumption of essential vitamins and minerals (i.e., those that can’t be synthesized by the body and must be obtained from diet). One of these essential minerals is iron, a critical cofactor for binding oxygen to several proteins in the body involved with oxygen transport and metabolism. In particular, iron is required for the production of hemoglobin (Hb), the protein in red blood cells that is responsible for binding oxygen and transporting it throughout the body. Given this critical role in facilitating aerobic respiration, a recent study examined how iron deficiency might impact exercise performance.
Iron deficiency and anemia
The body is capable of storing a certain amount of iron in the liver, spleen, muscle tissue, and bone marrow bound to the protein ferritin. Therefore, the development of iron deficiency requires a mismatch between iron loss (e.g., blood loss) and inadequate dietary intake, which, over a period of many months, can result in iron deficiency anemia – a condition characterized by an insufficient number of healthy red blood cells. Iron deficiency anemia (IDA) can have symptoms of increased heart rate, dizziness, cold hands and feet, and fatigue, especially with exercise.
In the earliest stages of deficiency, iron stores are depleted in order to continue the normal production of Hb and red blood cells. At this point, the individual is not yet anemic, as deficiency will not cause changes in Hb or hematocrit (a metric related to red blood cell count). However, declining serum ferritin levels reflect the gradual depletion of iron stores occurring at this stage. To compensate, the body will increase dietary iron absorption, which results in increased levels of serum transferrin levels (the protein responsible for ferrying iron throughout the body). One of the ways this change is measured is by transferrin saturation (TSat), the ratio between total serum iron and total iron binding capacity (the latter being an indirect metric of transferrin levels). If dietary iron remains low, serum iron levels and TSat will eventually start to decline. When TSat is less than 20%, it is indicative of iron deficiency, and when less than 16%, red blood cell (RBC) production becomes impaired. A diagnosis of anemia doesn’t occur until further iron depletion causes reduced Hb levels (<13 g/dL in men and <12 g/dL in women), despite a normal RBC appearance.
Studying ID in untrained individuals
Given the symptoms of fatigue associated with IDA, it is not surprising that intravenous (IV) iron supplementation has been shown to improve various exercise metrics among anemic athletes. But what about iron deficiency prior to the development of anemia? Studies in animal models have demonstrated that in iron deficiency without anemia (i.e., before iron deficiency causes a substantial decline in Hb levels), iron plays a critical role in response to hypoxia and can affect whole-body metabolism during exercise. Given these findings, researchers Frise et al. sought to determine how intravenous (IV) iron supplementation might impact exercise performance in untrained individuals who exhibited iron deficiency but did not reach the criteria for anemia.
In their prospective case-control study, the authors recruited cases and controls based on ferritin status from a pool of blood donors who were deemed to be iron deficient (ID) but not yet anemic. Iron deficiency was determined by the criteria of serum ferritin ≤15 μg/L and TSat <16%, whereas their iron-replete (IR) counterparts were required to have serum ferritin ≥20 μg/L and TSat ≥20%.
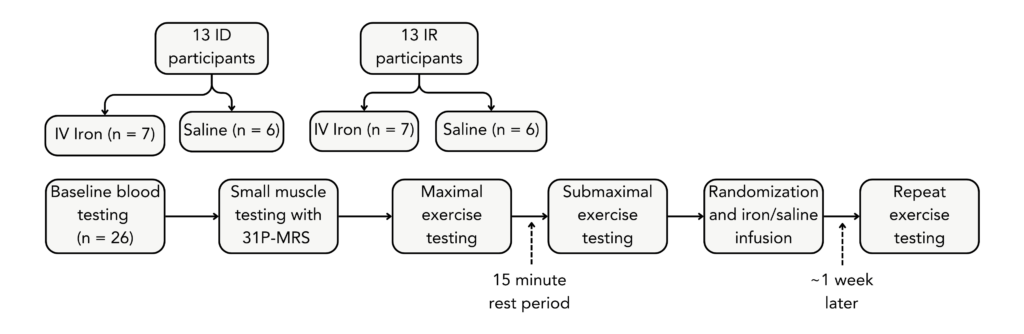
After completing a series of exercise tests at baseline, cases and controls were randomized ~1:1 to receive 15 mg/kg IV iron (up to a maximum of 1 g) or IV saline. Exercise assessment was repeated approximately one week later, a short enough interval after infusion that changes in Hb could not occur, such that metabolic changes could be separated from increases in oxygen delivery.
The exercise protocol included small muscle (calf) exercise tests, whole body maximal cardiopulmonary exercise testing (CPET, or VO2max testing), and submaximal exercise at 65% of VO2max. The calf exercises involved three 5-minute periods of plantarflexion exercise performed at 1 Hz at increasing levels of power: 3, 4, and 5 W, alternated with 7-minute recovery periods. At this level of intensity, the exercise is considered aerobic, and the calf muscle uses oxidative phosphorylation to generate the required energy. Oxidative phosphorylation is highest at rest and expected to drop during exercise regardless of iron status, but a greater drop in oxidative phosphorylation in the ID compared to the IR group would demonstrate metabolic impairment at low-intensity exercise. Thus, the authors used 31P magnetic resonance spectroscopy (MRS), an indirect measurement of mitochondrial oxidative phosphorylation, to image the calf during small muscle exercise tests.
Maximal exercise performance was measured using CPET on a cycle ergometer, with a venous blood sample taken in the final 30 seconds of each workload interval. After 15 minutes of recovery, a further 20 minutes of submaximal exercise was performed on the cycle ergometer, with the work rate (65% of VO2max) calculated from CPET results. Further blood samples were taken before starting and at 2-, 5-, 10-, 15-, and 20-minute time points during submaximal exercise.
What the study found
The mean baseline serum ferritin in the ID group (n=13, 12 female, 1 male) was 8.3 μg/L, and TSat was 10.6%. Despite not meeting Hb criteria for anemia (average Hb was 12.7 g/dL), these iron levels would suggest a depletion of iron stores and impaired RBC production. On the other hand, the mean serum baseline ferritin in the IR group was 58.0 μg/L, TSat was 35.2%, and average Hb was 13.6 g/dL.
Both the ID and IR participants who received an iron infusion had significant increases in serum ferritin and TSat but no change in Hb, whereas these metrics did not change among participants who received a saline infusion. After iron infusion, the mean serum ferritin in the ID group was 561±433 μg/L, a 68-fold increase, and TSat increased by more than 3.5-fold to 37.9±11.2%. In the IR group, iron resulted in a less dramatic 15-fold increase in mean serum ferritin to 840±331 μg/L and a 2.1-fold increase in TSat to 62.1±20.1%.
The ID and IR groups were found to differ significantly in exercise testing in a manner that was partially abolished with administration of IV iron to those who were iron deficient. Before IV iron, peak lactate at the end of CPET was the same in both groups, indicating equivalent anaerobic glycolytic capacity between groups. However, lactate clearance – an indication of how quickly a person can recover from this lactate accumulation while still exercising – was significantly slower in the ID group during the subsequent submaximal exercise. IV iron reduced peak lactate in both the ID and IR groups but accelerated lactate clearance only in the ID group (though not quite to the baseline clearance speed of the IR group). Additionally, the groups that received IV iron also had statistically significant improvements in two metrics measured by maximal exercise testing – a 10% increase in oxygen pulse (a measure of maximal aerobic capacity – VO2 divided by heart rate) and a 10% increase in lactate threshold (the transition point from slow to rapid lactate accumulation). Collectively, these results show that iron deficiency even in the absence of anemia negatively impacts exercise metrics, an effect which can be at least partially reversed with IV iron treatment.
31P MRS measurements of oxidative phosphorylation (the process for making ATP in the mitochondria) before or after iron infusions did not differ between ID and IR groups in the small muscle exercise test. This might suggest that the metabolic differences between the ID and IR groups are negligible at low exercise power, but it alternatively might reflect methodological limitations. 31P MRS is more typically used for detecting gross abnormalities of mitochondrial dysfunction rather than the subtle changes expected in this study, and the small sample size and noise inherent to the measurement may have restricted the ability to detect between-group differences whether or not they truly existed.
What might explain these findings
A lack of iron might affect skeletal muscle function and performance in several ways. As described above, iron is an essential cofactor of hemoglobin, which carries oxygen throughout the body. Likewise, a similar protein called myoglobin is responsible for carrying and storing oxygen within skeletal muscle and is similarly affected by insufficient iron stores. Iron deficiency therefore impairs hemoglobin- and myoglobin-mediated oxygen circulation, decreasing the ability of skeletal muscle to absorb oxygen. Not only does this cause the cardiovascular system to work harder to deliver oxygen to the muscle tissue, but without sufficient oxygen, ATP production shifts from oxidative phosphorylation (efficient) to anaerobic glycolysis (inefficient), which produces less ATP overall and increases fatigue, especially during exertion due to lactate and hydrogen accumulation.
Lactate threshold is a good predictor of the level of submaximal exercise that can be maintained over an extended time without fatigue. Below this threshold is generally considered aerobic exercise, where the majority of energy (as ATP) is generated from oxidative phosphorylation. The shift in lactate threshold after IV iron shows that iron deficiency promotes a shift away from oxidative phosphorylation and toward anaerobic glycolysis (leading to greater lactate accumulation) at a lower threshold of exercise intensity. No impairment of muscle oxidative phosphorylation was observed during small muscle testing, so it is presumed that the higher metabolic demands of intense exercise cause a whole-body shift in metabolism. Notably, since the IR group receiving IV iron also improved their lactate threshold and oxygen pulse, increased iron levels (within physiologic ranges) may improve lactate handling during exercise even in those without a deficiency.
In addition to promoting lactate production, iron deficiency also impairs lactate disposal, in which lactate is metabolized by other tissues, primarily the heart and liver. This effect on metabolism is mediated by mechanisms other than impaired oxygen delivery to these tissues, as IV iron altered lactate clearance without any change in Hb. Thus, it appears that a more direct role of iron in metabolism would explain the change, most likely through its involvement in the electron transport chain. Iron is a critical role cofactor in the class of proteins known as cytochromes, which are key components of the electron transport chain and thus of oxidative phosphorylation. If an individual is ID, cytochrome expression or activity may be lower, promoting a larger shift towards glycolysis, particularly as lactate accumulates during intense exercise. Iron deficiency may therefore reduce the efficiency of oxidative phosphorylation independently of its effects on oxygen delivery and Hb.
Who is most susceptible to ID
Although anyone with insufficient iron intake is susceptible to iron deficiency and IDA, certain populations are especially vulnerable. Compared to men and postmenopausal women (who need an average of 8 mg iron/day), premenopausal women need an average of more than double that amount at 18 mg to offset regular blood loss. In this study, the subjects were predominantly female (24 females, 2 males), which is representative of the higher prevalence of ID in premenopausal females – estimated between 17-57% depending on the diagnostic threshold used, compared to a much lower 4-8% of males.
Other factors that can make someone more susceptible to ID are a vegetarian or vegan diet, taking a proton pump inhibitor for heartburn, having celiac disease or other inflammatory bowel diseases, and being a competitive athlete. The dietary sources of iron in meat-free diets tend to have poor absorption since heme iron (from animal sources) is much more readily absorbed than non-heme iron (from plant sources). Proton pump inhibitors and gastrointestinal diseases reduce the absorption of dietary iron. Athletes are susceptible to iron deficiency due to increased hemolysis from high training loads and the impaired absorption of iron due to upregulation of the iron-regulating hormone, hepcidin, following exercise as part of the acute inflammatory response. Fortunately, low ferritin and ID are very treatable with iron supplementation, but this approach requires a level of caution since it can cause significant GI symptoms including constipation. Additionally, if someone is not low on iron, excess intake through supplements could eventually lead to iron overload (hemochromatosis). We will explore these variables and effects in more detail in an upcoming “Ask Me Anything” podcast episode.
The bottom line
Iron plays a critical role as a cofactor for proteins involved in oxygen transport and metabolism – two processes that are stressed by exercise. Long before one arrives at the doorstep of clinical anemia, exercise performance metrics are negatively affected by iron deficiency due to compensatory metabolic changes. Individuals with low ferritin will often have some of the same symptoms of fatigue as those with a diagnosis of IDA, and repleting iron stores can improve not only their overall well-being, but their exercise capacity as well. However, before beginning an iron supplement, it is important to confirm low ferritin levels with a blood test. In the absence of an obvious cause of iron loss (such as heavy menstrual periods or a recent change to a vegetarian or vegan diet), it is vital to have subsequent tests to determine the cause of low ferritin, so that supplementing doesn’t mask an underlying condition. For instance, low iron in an individual over the age of 50 should be assumed to be colon cancer until proven otherwise. So, identification of cause – low intake, low absorption, or iron loss – must be established.
I will dive deeper into iron deficiency in the upcoming AMA, but generally, the best course of action when faced with low iron levels depends on the cause. For those who have low iron simply due to insufficient intake, increasing dietary iron intake is effective, and taking daily iron supplements is a low-cost option for those who cannot get adequate intake to replete iron stores through diet. This strategy also works for some cases of low iron absorption or even for iron loss when the root cause is not pathologic – such as blood loss from chronic phlebotomies or menstruation.
However, in some cases, low iron due to impaired iron absorption or excess iron loss reflects underlying causes which require other courses of action. For example, a helicobacter pylori infection will reduce oral iron absorption from diet or supplements, and thus, treating the infection is required to fully remedy the situation. Other causes of reduced intestinal absorption – including gastric bypass surgery, inflammatory bowel disease, and malabsorption syndromes such as celiac disease – may require intravenous iron to circumvent the gastrointestinal tract entirely. Likewise, in cases of iron loss due to pathologic blood loss, the root cause must be identified and treated.
Iron deficiency without anemia is likely widely underdiagnosed and may be due to a variety of potential causes. Being attentive to iron levels is therefore not only important due to the effects of iron itself, but also as an indicator of other aspects of whole-body health. By identifying and treating the causes of low iron and increasing iron stores, we can improve exercise performance, as well as many other elements of overall health and well-being.
For a list of all previous weekly emails, click here.