Each year, approximately 264,000 new cases of breast cancer are diagnosed in women in the United States, accounting for 30% of all new cancer cases among women. Over the past thirty years, modest strides have been made in reducing breast cancer mortality, largely due to increased and improved screening and more effective treatments.
Prognosis is dependent on both how advanced the disease is at the time of diagnosis and the characteristics of the tumor. Breast cancer is categorized by the presence or absence of several types of receptors on the cell membrane: estrogen, progesterone, and human epidermal growth factor receptor 2 (HER2). When caught early, the best survival rates occur with hormone receptor-positive, HER2-negative tumors. Survival rates are lower with tumors enriched with HER2 receptors due to the resistance to anti-HER2 treatments that occurs either de novo or over the course of treatment. The worst prognoses occur in tumors that do not express any of these three types of receptors, also known as triple-negative breast cancer (TNBC), as shown in the table below. The lack of receptor expression in TNBC makes it difficult to use targeted treatments to kill these tumor cells, but research suggests inspiration for a potential new, effective treatment may come from an unlikely source – a bee sting.
Table: Breast cancer five-year survival adapted from Chavez-Macgregor et al. 2017 and Waks and Winer 2019.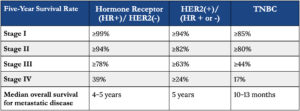
Why it might work
The pain from a honeybee sting is largely caused by a peptide known as melittin – the main component of bee venom (BV) – which directly activates nociceptors, the sensory receptors that initiate the sensation of pain. Melittin is also toxic to nearby cells, as it forms pores in local cell membranes and cell matrices, which in turn triggers an inflammatory response. These pore-forming, cytotoxic effects underlie interest in melittin as a potential cancer treatment – if it can selectively target cancer cells. Indeed, researchers Duffy et al. have found that both BV and melittin potently induced cell death in TNBC and HER2-enriched cell types and had a higher potency in cancer cells compared to normal cells.
The anti-cancer specificity of melittin is due to its affinity for attaching to the plasma membranes of cells overexpressing epidermal growth factor receptors (EGFR), a characteristic in 50% of TNBC subtypes. Once attached to the cell membrane, melittin suppresses the activation of EGFR and HER2, which results in the inhibition of PI3K/Akt signaling, a major pathway controlling cell proliferation (for a quick refresher on this, check out the segment starting at 1:28:00, from my interview with Lew Cantley, who led the team that discovered PI3K). By inhibiting cell proliferation, melittin slows tumor growth and sensitizes the tumor to other treatments. Even though TNBC has a high frequency of overexpressing EGFR, previous anti-EGFR therapies have been largely unsuccessful. The in vitro success of melittin as an anti-EGFR therapy shows promise for translation to future TNBC clinical trials.
Despite its affinity for cancer cells, natural melittin, like other anti-cancer substances found in nature, is associated with significant off-target toxicity in normal cells. This is always the problem with chemotherapy–it’s not the killing cancer part that’s hard; it’s the killing cancer while not killing non-cancer that’s hard. However, modification of one end of the melittin amino acid chain has been shown to enhance cancer cell selectivity. By augmenting this region of the molecule with a peptide that interacts with cell adhesion receptors commonly overexpressed in breast cancer cells and tumor vasculature, scientists can engineer a form of melittin with better selectivity and lower off-target toxicity than observed with the natural compound.
What the study found
The study by Duffy et al. investigated the dose-response effect of BV and pure melittin on cell viability in eleven different cell lines: 3 normal breast, 3 TNBC, 2 HER2-enriched, and 3 luminal breast cancer cell lines. The dose required to induce 50% cell death was significantly lower in all three types of cancer cell lines than in normal breast cells. The authors then used the dose for each of the three cell lines that induced 50% cell death to assess the viability over time and the mechanism of cell death. After a 60-minute treatment with melittin, TNBC and HER-2 enriched cell lines had 20% and 40% viability, respectively, compared to 80% of normal breast cells. The researchers confirmed that cell death was predominantly caused by membrane disruption, consistent with melittin’s mechanism of action.
Since most chemotherapy regimens use a combination of anti-cancer pharmaceuticals rather than a single agent, an additional arm of the study evaluated the efficacy of a combination of melittin and the chemotherapeutic docetaxel when injected into the tumor of a TNBC mouse model. The mice treated with melittin and docetaxel were found to have smaller tumor volume after 14 days than mice treated with either treatment alone. In addition to membrane receptor disruption, the melittin-induced pore formation in the cell membrane enabled the internalization of other molecules such as chemotherapeutic drugs. In other words, melittin sensitized TNBC cells to other chemotherapeutics in vivo, creating a synergistic antitumor response with docetaxel that was more effective than either agent alone.
Part of this response is likely due to melitttin’s inhibition of cell proliferation and membrane pore creation, but there may be additional benefits of inhibiting EGFR and HER2 signaling. Treatment with melittin also reduced tumor expression of programmed death ligand-1 (PD-L1). PD-L1 is an immune-checkpoint protein that slows immune response by reducing the functionality of activated T-cells. Reduced levels of PD-L1 prevented the tumor cells from developing adaptive immune resistance, further increasing therapeutic efficacy over chemotherapy alone. (For more information on immunotherapy and how the immune system helps to battle cancer, check out my podcast interview with Dr. Steven Rosenberg.) Among breast cancer subtypes, PD-L1 is expressed most highly in TNBC tumors, followed by HER2-enriched tumors. The addition of an anti-PD-L1 therapy to adjuvant chemotherapy increased the number of patients with TNBC who experienced a complete pathological response by 13.6 – 14.8% over chemotherapy alone. Although the overall survival from this new treatment is still being monitored, for the 30-40% of patients who don’t experience a complete pathological response, the development of new treatments such as modified melittin might be another avenue for treatment.
Next steps
This isn’t the first time honeybee venom and melittin have been considered as candidates for cancer therapy, but neither has gotten much clinical traction due to melittin’s nonspecific cytotoxicity and instability. Honeybee venom is designed to cause local cytotoxicity, resulting in the pain and inflammation of a bee sting. If these cell-damaging effects can be controlled and directed toward a tumor, they may be the key to creating a potent cancer treatment. So what’s next when it comes to developing viable melittin-based treatments?
Melittin, even if modified, will likely need a carrier to allow for safe introduction into circulation. If injected intravenously, melittin causes hemolysis, a severe reaction that destroys red blood cells through membrane rupture. Obviously a nonstarter. The development of a carrier is essential to translating melittin from cell and localized animal studies into clinical studies, which would require intravenous infusions. Already some studies have successfully used targeted polymeric nanoparticles to systemically carry melittin to treat a metastatic breast cancer model in mice.
Once the kinks have been worked out, melittin may have applications beyond breast cancer, as other types of cancer also overexpress EGFR and HER2. Regardless of cancer type, overexpression of EGFR is increasingly recognized as a biomarker of treatment resistance and overexpression of HER2 is likewise associated with poor outcomes. Melittin with cancer-specific modifications may be able to kill tumor cells, reduce immune evasion, and enhance the effects of other adjuvant chemotherapeutics.
In a larger sense, melittin is a proof of principle that natural compounds, such as venoms and plant toxins, can offer promising avenues for cancer therapies. If the mechanisms by which natural compounds cause cell death can be engineered with modifications to have high cancer specificity, there is potential for new and potent cancer treatments, inspired by peptides found in nature.
For a list of all previous weekly emails, click here.




