Earlier this month, we released a podcast I’d recently recorded with Dr. JoAnn Manson, a principal investigator for the Women’s Health Initiative (WHI), on the risks and benefits of hormone replacement therapy (HRT). But in the days following the episode’s release, it became clear that it raised as many questions as it answered and heightened, rather than reduced, confusion and anxiety over HRT.
My goal for all of my podcast discussions is to leave listeners with deeper understanding and a greater confidence in their ability to make health-related decisions, so if I’ve fallen short of that this time, allow me now to offer better clarity.
A brief refresher on context
When women go through menopause, levels of the sex hormones estrogen and progesterone plummet, causing all of the vasomotor symptoms (e.g., hot flashes and night sweats) classically associated with this period of a woman’s life. These hormonal changes also result in losses in bone mineral density and muscle strength, changes in mood, decline in libido and sexual function, and various other effects impacting quality of life and physical health. Hormone therapy is intended to attenuate these undesirable symptoms of menopause by providing exogenous estrogen (with or without progesterone) to supplement the body’s low levels.
Observational studies in the 1980s and 90s had indicated that HRT might also reduce risk of cardiovascular disease (CVD) and cognitive decline, which provided the motivation for Dr. Manson and her fellow researchers to begin the Women’s Health Initiative – two randomized clinical trials (one testing estrogen alone and the other testing estrogen plus a synthetic form of progesterone) designed to investigate the efficacy of HRT for chronic disease prevention. But when the WHI’s results were first published in 2002, the investigators reported no reduction in heart disease among those on HRT, but slightly increased incidence of breast cancer. The media jumped on these findings, and use of HRT – which until then had been the standard of care for menopausal symptoms – plunged by 70-80%.
Since then, many have pointed out that the risks were very overblown, and Dr. Manson herself expressed her belief that these results should not be extrapolated to the point of denying HRT to women in early menopause for symptom relief. Still, debate continues to rage over the magnitude of risks and benefits – and over exactly who should be on HRT and for how long.
On what do we all agree (and disagree)?
Let me be very clear: HRT is unquestionably the single most effective available treatment for easing the symptoms of menopause. Despite the WHI’s reports and subsequent alarmist media coverage, no one disputes HRT’s efficacy in relieving menopausal symptoms and thereby improving quality of life. Rather, the debate lies in whether this relief comes at a cost of raising one’s risk of certain chronic diseases, particularly breast cancer, dementia, and cardiovascular disease.
Which brings me to another point of broad consensus in the medical community: HRT should only be initiated within the first 10 years of the onset of menopause. Starting hormone therapy more than a decade after menopausal onset means experiencing a prolonged period of estrogen deprivation, which likely diminishes any potential benefits of HRT and may increase any potential risks. For instance, a 2004 meta-analysis of randomized trials reported that women enrolled in hormone therapy trials before age 60 were at significantly reduced all-cause mortality risk relative to placebo (OR: 0.61, 95% CI: 0.39-0.95, but this apparent benefit was absent in women enrolled over the age of 60 (OR: 1.03, 95% CI: 0.90-1.18). Risk of dementia on HRT – as discussed in more detail below – also may be reduced in those who start treatment during early menopause but may increase in those who begin treatment late. However, this concern only applies to initiating HRT more than 10 years after menopausal onset, which should not be confused with continuing HRT beyond 10 years after menopausal onset.
Whether or not to continue HRT post-menopause is another point of debate. Some believe that, regardless of the time of initiation, hormone therapy comes with an increased risk of disease and is only warranted for cases in which symptoms of menopause are severe enough to justify increased risk. These individuals therefore advocate against continuing HRT after menopausal symptoms have subsided, as any other possible benefits are insufficient to offset the risks. Others – myself included – maintain that hormone therapy has substantial positive impacts beyond menopausal symptom relief and that the alleged risks associated with HRT are not supported by evidence, lack clinical significance, or otherwise fail to outweigh the benefits to health and quality of life.
In other words, the decisions to initiate HRT or continue post-menopause boil down to a risk-benefit analysis, and the best choice may vary across individuals based on factors such as menopausal symptom severity, family history, and personal preferences. So while I can’t offer a generic, one-size-fits-all answer, I can share what we currently know about the benefits and risks of HRT so that you can make informed decisions for yourself or your patients.
How does HRT impact risk of breast cancer?
First, let’s discuss the foremost concern raised by the WHI: breast cancer.
The WHI reported increased incidence of breast cancer in the group given conjugated equine estrogen (CEE) plus the progestogen medroxyprogesterone acetate (MPA), the dominant form of HRT used in the 1990s, relative to controls on placebo (HR: 1.24, 95% CI: 1.02-1.50). This difference, which raised alarm bells among the popular press, corresponded to about one additional case of breast cancer per 1,000 patient-years, and the discrepancy disappeared altogether when considering only women with no hormone therapy prior to the study (most of the women). This latter analysis also revealed that the apparent increase in risk was not due to an unusually high incidence in those on CEE + MPA, but an unusually low incidence in those on placebo who had previously used HRT (see Figure 1 below). The parallel study, comparing placebo versus CEE alone, showed no increased breast cancer risk with treatment, and indeed, the CEE alone (no MPA) group was found to have nearly a 20% lower risk than the placebo group (Figure 2).
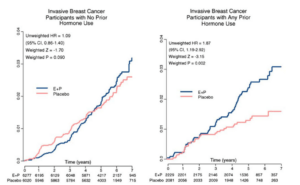
Figure 1: Women’s Health Initiative data on breast cancer incidence on estrogen plus progestin versus placebo, divided based on whether participants had (right) or had not (left) used hormone therapy prior to the study start. Note the low incidence of breast cancer in women on placebo with prior hormone use. From Anderson et al. 2006.
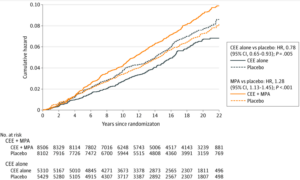
Figure 2: Breast cancer incidence with estrogen alone (solid navy line) vs. placebo (dashed navy line) and with CEE + MPA (solid orange line) vs. placebo (dashed orange line) in 22-year follow-up of the Women’s Health Initiative cohorts. From Chlebowski et al. 2020.
Critically, even for women on CEE + MPA, deaths from breast cancer were not significantly increased relative to women on placebo, despite the very small increase in breast cancer incidence. The data showed a non-significant trend toward higher mortality, though this was likely attributable to the increase in breast density with treatment, making lesions more difficult to detect. This is less of a concern today, as screening methods and recommendations have improved considerably over the last 20 years. Further, the estrogen-only group had a 40% reduction in breast cancer mortality relative to placebo by 20 years of follow-up, a difference which achieved statistical significance.
I want you to read this again, because there may be no greater lie told to women than this one: based on the WHI we “know” estrogen causes breast cancer. This is categorically untrue. But, as Mark Twain said, “a lie can travel halfway around the world before the truth puts on its shoes.” I’d be hard pressed to find a better example of this phenomenon in modern medicine.
The opposing results with estrogen only versus estrogen + MPA strongly suggest that MPA, and not estrogen, is responsible for any elevated risk observed in the WHI and that estrogen itself may in fact be protective. Since MPA is no longer used in HRT (as a synthetic hormone it fell out of favor and has been replaced by bioidentical micronized progesterone for women requiring systemic progesterone or progesterone-coated IUDs for those only needing endometrial protection), the WHI’s results on breast cancer are effectively outdated. However, data from trials using newer HRT formulations – particularly transdermal estradiol and micronized progesterone – don’t yet exist and likely never will, so we do not know if and how these formulations would affect breast cancer risk.
How does HRT impact bone health?
Estrogen is an essential hormone for maintaining bone density, as it both inhibits bone resorption and is part of the signaling pathway by which mechanical stress (i.e., resistance exercise) triggers formation of new bone tissue. Therefore, when estrogen levels drop dramatically during menopause, the rate at which bone is eaten away increases, and at the same time, resistance training becomes less effective in stimulating bone regrowth. This combination results in significant loss of bone mineral density (BMD), often to the level of osteoporosis. Since HRT keeps estrogen levels from dropping too low, it prevents this loss and thus lowers risk of potentially debilitating fractures, as shown in the WHI itself (and discussed in more detail in “Ask Me Anything” episode #37 on bone health).
As soon as HRT is stopped, the protection it provided against loss of BMD disappears. But even though bone loss will occur after ceasing HRT, simply delaying this loss means maintaining healthy bone mass over a longer period of time and pushing back the time it will take for bones to devolve to a state of osteoporosis. If two women lose bone mass at the same rate, but Woman A starts declining at age 50 and Woman B at age 70, Woman B will have much stronger bones at age 80 than the woman who had a 20-year headstart on bone deterioration.
Importantly, osteoporosis medications such as bisphosphonates can also slow bone loss. However, these drugs work by interfering with resorption mechanisms and, unlike HRT, do not address the underlying cause of impaired signaling for bone remodeling – the loss of estrogen. Replacing estrogen levels can restore the ability of resistance training to stimulate bone formation, effectively giving a woman a longer window over which she might maintain or improve BMD with weightlifting, such that she is starting from a better position once bone mass begins to decline.
How does HRT impact risk of dementia?
The WHI also reported higher rates of probable dementia with both estrogen alone (HR: 1.24, 95% CI: 0.83-2.66) and CEE + MPA (HR: 2.05, 95% CI: 1.21-3.48) relative to placebo groups, though this elevation did not reach significance for estrogen-only treatment. Yet as the authors themselves note, age at which HRT is initiated may be a critical factor in this apparent risk.
This analysis of WHI data was limited to women ages 65-79 at baseline, a range which we can safely assume is long after the onset of menopause. A little over half (~55%) of the women in the estrogen-only group had never used hormone therapy prior to the study, meaning that their first experience with HRT would have followed several years of estrogen deprivation – as discussed earlier, practitioners generally advise against this. Indeed, when the analysis was restricted only to those with no prior hormone use, hazard ratios with HRT were higher than in the full cohort (HR: 1.95, 95% CI: 0.94-4.04). Likewise, when the analysis was restricted to those who had previously taken HRT, any apparent risk elevation disappeared completely (HR: 0.87, 95% CI: 0.32-2.39). Sub-analyses of estrogen + MPA data yielded nearly identical results.
These findings on age dependence agree with an observational study which stratified women into four groups based on whether HRT was taken: (1) only in mid-life (mean age: 48.7), (2) only in late-life (mean age: 76), (3) through both mid- and late-life, or (4) never. Similar to the WHI, the investigators found that HRT was only associated with an elevated risk of dementia when started late in life relative to those who had never used hormones (adjusted HR: 1.48, 95% CI: 1.10-1.98). For those on HRT only during mid-life, the therapy actually had a significant protective effect (adjusted HR: 0.74, 95% CI: 0.58-0.94), and no difference in risk was observed between those who had never used HRT and those who started in mid-life and continued through late-life (adjusted HR: 1.02, 95% CI: 0.78-1.34). Collectively, these findings certainly reinforce the notion that HRT should not be started more than 10 years after menopause onset, but importantly, for those who initiate early, hormone therapy does not increase dementia risk, even if treatment continues for many years after menopause symptoms subside.
How does HRT impact risk of cardiovascular disease?
Menopause is associated with the development of a range of risk factors for CVD, including increased visceral fat, reduced glucose tolerance, hypertension, and dyslipidemia. The drop in estrogen occurring during the menopausal transition causes fat tissue to redistribute from subcutaneous depots to the visceral space, which may in turn lead to insulin resistance, dyslipidemia, and other effects which raise CVD risk.
By replacing the estrogen deficit, hormone therapy could theoretically ameliorate some of these risks. This concept has been supported by large-scale epidemiological studies, most notably the Nurses’ Health Study, in which women with no history of heart disease (n=48,470) were found at a 10-year follow-up to have a 44% reduction in risk of major coronary disease when taking estrogen (95% CI: 20-60%). Age-adjusted risk for cardiovascular mortality was 32% (95% CI: 10-48%) lower in those who had been on HRT.
In looking at data from the WHI, evidence of HRT’s potential to reduce CVD mortality risk seems less compelling. The study revealed no significant decrease (or increase) in CVD mortality for either of the two HRT formulations in either the initial dataset or at the latest follow-up point (data through 2016), suggesting that HRT may not be harmful to cardiovascular health, but also doesn’t appear beneficial.
However, the age at which HRT is initiated may again influence these results. A secondary analysis of pooled data from both WHI trials found that, among women who began the study within 10 years of starting menopause, HRT was associated with reduced coronary disease (HR: 0.76, 95% CI: 0.50-1.16) relative to placebo, though not to statistical significance. In contrast, for women who initiated hormone therapy more than 20 years after menopause onset, HRT was associated with a significant increase in CVD risk (HR: 1.28, 95% CI: 1.03-1.58), while those 10-19 years post-menopause showed an intermediate and non-significant risk. This trend indicates that when it comes to HRT, starting early reduces potential cardiovascular risks and may even provide additional CV protection.
Additionally, outdated formulations may once again be an important factor. Both treatment groups in the WHI trials were taking CEE, an oral estrogen formulation. Such formulations are known to slightly increase blood viscosity, which in turn elevate cardiovascular risk. But oral estrogens are rarely used today, as they have largely been replaced by topical formulations which do not have the same effects on blood viscosity.
What can we conclude?
Careful consideration of existing data reveals that the widespread panic over hormone therapy risks has not been remotely justified. Yet, tragically, literally millions of women today have been and continue to be denied this therapy by doctors who simply fail to study the results of the very same trial they use to lambast hormones. Results from the WHI and other studies offer no indication that HRT poses any meaningful threat when initiated early in menopause; on the contrary, it likely provides protection from chronic diseases such as breast cancer, dementia, and heart disease, in addition to its clear benefits for bone health and frailty prevention. While these data also show us that risks may increase significantly when HRT is started a decade or more after menopause onset, they do not demonstrate similar risk in continuing HRT post-menopause.
Unfortunately, most available data (including from the WHI) investigated HRT formulations which are now all but obsolete and conducted trials in which interventions lasted only a few years. Randomized trials have not been published using newer iterations like transdermal estradiol and micronized progesterone, so we can’t be sure if risks and benefits associated with older formulations apply to those most commonly used today. We also lack clinical trial data on long-term HRT use, even for the formulations used in the WHI, and such results will likely never exist due to the high costs and difficult logistics of decades-long trials.
Some view this uncertainty as reason to avoid HRT altogether, but as I’ve expressed many times in the past, certainty is an unattainable goal in science and medicine, and the best we can do is to weigh risks and benefits based on the evidence at hand and make a choice that has the highest probability of a positive outcome. The information gained by studying earlier HRT formulations provide some of the best-available clues as to the effects of those in use today, and the cost of waiting for future trials to achieve a somewhat higher level of confidence must be weighed against the number of patients whose lives and health might be improved by starting or continuing HRT now. Likewise, we don’t have long-term randomized trial data on virtually any chronic medications used today, but should we deny patients life-saving drugs such as PCSK9 inhibitors or antihypertensive medications simply because no one will ever conduct an exorbitantly expensive 30-year clinical trial? Absolutely not.
So again, when it comes to HRT or any other therapeutic intervention, there are always risks and benefits. How they balance may depend on the individual patient, but to my eyes, the scale tips in favor of HRT more often than it tips against it. The reports of health risks are dubious at best, whereas the boons to menopause symptom relief, bone health, psychological and sexual well-being, and possibly risk mitigation for chronic diseases all have the potential to increase one’s chances of living healthier, happier, and longer.
For a list of all previous weekly emails, click here.




