Want to catch up with other articles from this series?
- The straight dope on cholesterol – Part I
- The straight dope on cholesterol – Part II
- The straight dope on cholesterol – Part III
- The straight dope on cholesterol – Part IV
- The straight dope on cholesterol – Part V
- The straight dope on cholesterol – Part VI
- The straight dope on cholesterol – Part VII
- The straight dope on cholesterol – Part VIII
- The straight dope on cholesterol – Part IX
Previously we addressed these 3 concepts:
#1 — What is cholesterol?
#2 — What is the relationship between the cholesterol we eat and the cholesterol in our body?
#3 — Is cholesterol bad?
I want to thank folks for doing their best to resist the following two urges:
- Please resist asking me questions beyond the scope of this post. If it’s not in here, it will probably be in a subsequent post in this series.
- Please resist sending me your cholesterol numbers. Share your story with me and others, but understand that I can’t really comment other than to say what I pretty much say to everyone: standard cholesterol testing (including VAP) is of limited value and you should have a lipoprotein analysis using NMR spectroscopy (if you don’t know what I mean by this, that’s ok… you will soon). I can’t practice medicine over the internet.
Remember last week’s take away messages:
- Cholesterol is “just” another fancy organic molecule in our body but with an interesting distinction: we eat it, we make it, we store it, and we excrete it – all in different amounts.
- The pool of cholesterol in our body is essential for life. No cholesterol = no life.
- Cholesterol exists in 2 forms – unesterified or “free” (UC) and esterified (CE) – and the form determines if we can absorb it or not, or store it or not (among other things).
- Much of the cholesterol we eat is in the form of CE. It is not absorbed and is excreted by our gut (i.e., leaves our body in stool). The reason this occurs is that CE not only has to be de-esterified, but it competes for absorption with the vastly larger amounts of UC supplied by the biliary route.
- Re-absorption of the cholesterol we synthesize in our body (i.e., endogenous produced cholesterol) is the dominant source of the cholesterol in our body. That is, most of the cholesterol in our body was made by our body.
- The process of regulating cholesterol is very complex and multifaceted with multiple layers of control. I’ve only touched on the absorption side, but the synthesis side is also complex and highly regulated. You will discover that synthesis and absorption are very interrelated.
- Eating cholesterol has very little impact on the cholesterol levels in your body. This is a fact, not my opinion. Anyone who tells you different is, at best, ignorant of this topic. At worst, they are a deliberate charlatan. Years ago the Canadian Guidelines removed the limitation of dietary cholesterol. The rest of the world, especially the United States, needs to catch up. To see an important reference on this topic, please look here.
Concept #4 – How does cholesterol move around our body?
To understand how cholesterol travels around our body requires some understanding of the distinction between what is hydrophobic and hydrophilic. A molecule is said to be hydrophobic (also called nonpolar) if it repels water, while a molecule is said to be hydrophilic (also called polar) if it attracts water. I could spend a lot of time getting in to the nuances of these properties, but I think it’s best to just focus on the major issues. Think of your veins, arteries, and capillaries as the “waterways” or rivers of your body.
BONUS concept: Another important concept is that cell membranes are lipid bilayers (which are hydrophobic) as I wrote about last week. Hence, a hydrophilic substance cannot pass through lipid membranes. Substances that can pass through lipid membranes are said to be lipophilic. A substance that has both polar (hydrophilic) and nonpolar (hydrophobic) properties is called amphipathic. The fact that unesterified cholesterol (UC) is an amphipathic molecule is a crucial property for its location in cell membranes. CE in which the –OH group has been replaced by a long chain fatty acid is a very nonpolar or hydrophobic molecule.
If a molecule needs to travel from your gastrointestinal tract (A) to, say, a cell in your quadriceps muscle (B) it needs to get on the river and travel from point A to point B. Because blood is effectively water, (the “water” part of blood is called plasma, an aqueous solution with a bunch of “stuff” in it (e.g., red blood cells, white blood cells, other proteins, ions) there are two ways to move down the river – swim or hitch a ride on a boat.
If a molecule is hydrophilic, it can be transported in our bloodstream without any assistance – sort of like swimming freely in the river – because it is not repelled by water. Conversely, if a molecule is hydrophobic, it must have a “transporter” to move about the river because the plasma (water) wants to repel it. I know this seems like a strange concept, but if you think about it, you’ve already seen great examples in your day-to-day life:
Sugar and salt will easily dissolve in water. They are, therefore, hydrophilic. Oil does not dissolve in water. It is, therefore, hydrophobic.
By extension, a molecule of glucose (sugar) or sodium and chloride ions (salt), because of their chemical properties which I won’t detail here, will travel through plasma without assistance. A lipid will not.
All of this is a long way of saying that sterol lipids (of which cholesterol ester is the predominant form in plasma), because they are hydrophobic, need to be carried around our bloodstream. They can’t move from one place to the next without a protein transporting molecule.
In other words, cholesterol doesn’t exist in our bloodstream without something to carry it from point A to point B.
So what are these “transporting molecules” called?
The proteins that traffic collections of lipids are called apoproteins. Once bound to lipids they are called apolipoproteins, and the protein wrapped “vehicle” that transports the lipids are called lipoproteins. Many of you have probably heard this term before, but I’d like to ensure everyone really understands their important features. A crucial concept is that, for the most part, lipids go nowhere in the human body unless they are a passenger inside a protein wrapped vehicle called a lipoprotein. As their name suggests lipoproteins are part lipid and part protein. They are mostly spherical structures which are held together by a phospholipid membrane (which, of course, contains free cholesterol). The figure below shows a schematic of a lipoprotein.
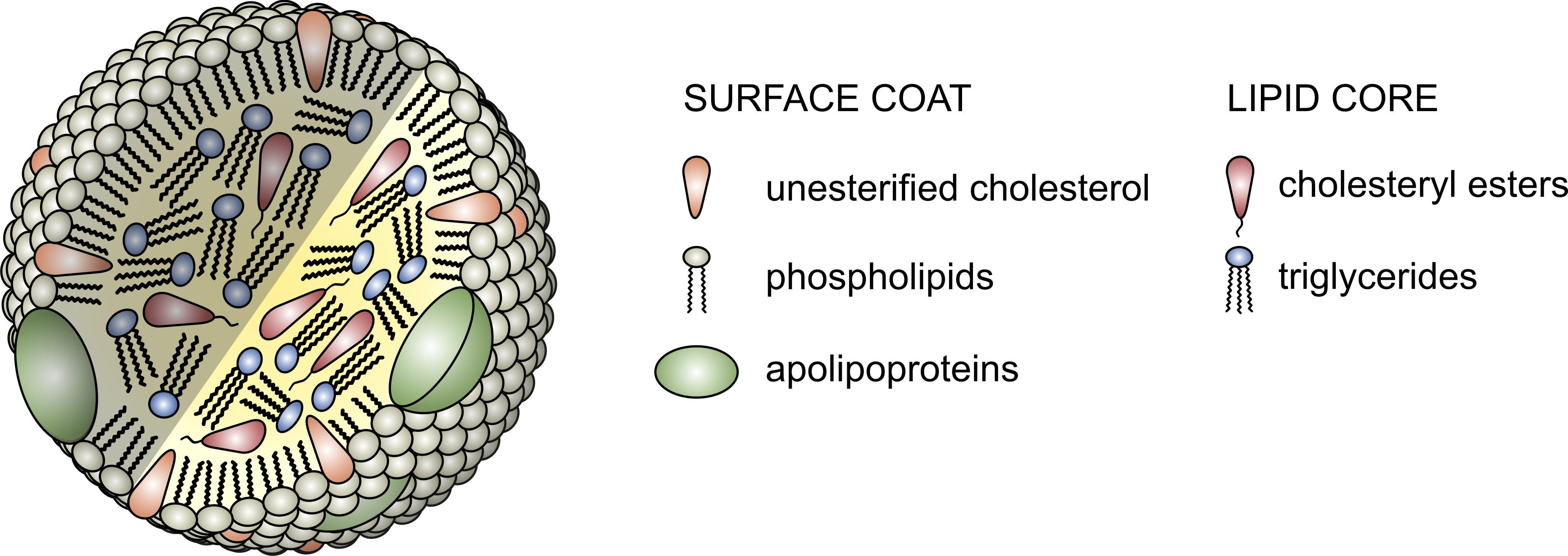
- Assisting in the structural integrity and solubility of the lipoprotein;
- Serving as co-factors in enzymatic reactions;
- Acting as ligands (i.e., structures that help with binding) for situations when the lipoprotein needs to interact with a receptor on a cell.
Apolipoproteins come in different shapes and sizes which determine their “class.” Without getting into the details of protein structure and folding, let me focus on two important classes: apolipoprotein A-I and apolipoprotein B. Apoprotein A-I (abbreviated apoA-I), which is composed of alpha-helicies, form lipoproteins which are higher in density. (The “A” class designation stems from the fact that apoA’s migrate with alpha-proteins in an electrophoretic field). Conversely, apoprotein B (abbreviated apoB), which is predominantly composed of beta-pleated-sheets, form lipoproteins which are lower in density. (The “B” class designation stems from the fact that apoB’s migrate with beta-proteins in an electrophoretic field.)
Virtually all apoB in our body is found on low-density lipoprotein – LDL, while most apoA-I in our body is found on high-density lipoprotein – HDL. Going one step further, the main structural apoprotein on the LDL is called apoB100 (though we often shorten this to just “apoB”), and there is only one apoB molecule per particle. It’s starting to come together now with “high” and “low” density lipoproteins, isn’t it?
But there’s actually more to it.
Everything I just described above deals with the structure and surface of the lipoprotein molecule – sort of the like the hull of the ship. But, what about the cargo? Remember what started this discussion. It’s all about transporting cholesterol (and lipids) which can’t freely travel in the bloodstream. The “cargo” of these ships, what they actually carry both on their surface [molecules of cholesterol and phospholipids] and in their core [cholesteryl esters (CE) and triglycerides (TG, or triacylglycerols)] is what we’ll now turn our attention to.
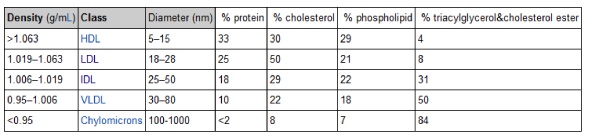
The ratio of lipid-to-protein in the lipoprotein structure determines its density – which is defined as mass per unit volume. Something that has a high density is heavier for a given volume than something with a low density. The table in this link (which I’ve also included below) shows the relative density of the five main classes of lipoproteins (from most dense to least dense) as they were originally discovered using ultracentrifugation: high density lipoprotein (HDL), low density lipoprotein (LDL), intermediate density lipoprotein (IDL), very low density lipoprotein (VLDL), and chylomicron.
Note the very subtle difference in density between the most and least dense lipoprotein – about 10 or 15%. Conversely, note the very large difference in diameter between each lipoprotein – as much as 2 orders of magnitude. Later in this series, when we start to talk about the volume of a lipoprotein particle, this difference will be amplified 1,000 times (because volume is calculated to the third power of diameter).
Below is a figure I’ve borrowed graciously from one of Tom Dayspring’s remarkable lectures which gives you a sense of the diversity of each of these classes of lipoproteins as well as the subclasses within each class. If this topic wasn’t confusing enough, there are actually multiple nomenclatures for the HDL subparticles. Originally, nomenclature was based on their buoyancy. Today nomenclature is based on the following methods, dependent on the technology used to measure them:
- Particle separation using gradient gel electrophoretic fractionation (deployed by Berkeley Heart Lab).
- Magnetic resonance assaying of lipid terminal methyl groups, called Nuclear Magnetic Resonance, or NMR (deployed by Liposcience).
- Two-dimensional gradient gel electrophoresis and apoA-I staining (deployed by Boston Heart Lab).
We’ll cover this later, but I want to point this out now to avoid (unnecessary) confusion in the figure below, which uses the first two of these.
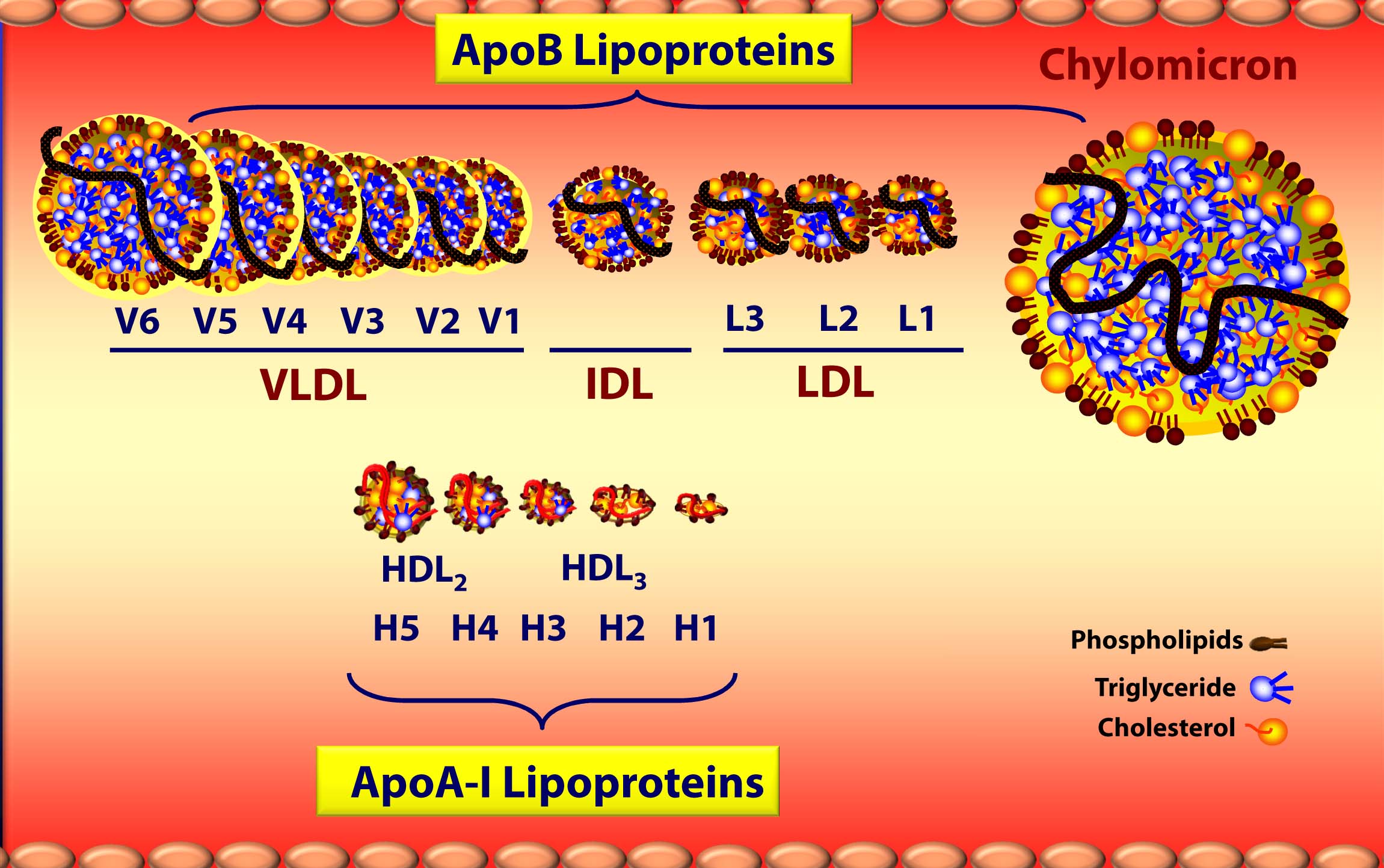
A few things probably jump out as you look at this figure:
- ApoA-I lipoproteins (i.e., HDLs) are tiny compared to ApoB lipoproteins (i.e., VLDL’s, IDL’s, and LDL’s) [this figure is not actually to scale – the “real” difference is even more pronounced.]
- As a general rule (with pathological exceptions), as particles move from being larger to smaller, the relative content of triglycerides (TG) goes down while the relative content of protein goes up, hence the density change.
- Actual cholesterol mass is greatest in the LDL particle.
- Each specific lipoprotein has a different core make up – meaning the variable ratio of TG to cholesterol ester changes. A particle of VLDL has 5 times more TG than CE whereas a particle of LDL typically has 4 or more times more CE than TG (i.e., ratio > 4:1), and an HDL has 90-95% CE and < 10% TG in its core.
- The TG trafficking lipoproteins are chylomicrons from the intestine and VLDLs from the liver.
Deep breath. Anyone left wondering why this topic is NOT covered in medical school? I think I can conservatively say 95% to 99% of physicians do not know what you have just learned — not because they aren’t “smart,” but because this topic is simply not covered in medical school, and the pace at which the field is developing is too great for most doctors to keep up with.
Why is cholesterol concentration increasing and triglyceride concentration decreasing as lipoproteins progress from larger to smaller?
The liver exports VLDL which, after chylomicrons (used to get triglycerides to muscles and adipocytes and cholesterol from the gut to the liver) are the largest of the lipoprotein particles. VLDL particles “give up” some of their triglycerides in the form of free fatty acids and shrink as they also release surface phospholipids. Once a certain size or buoyancy is reached it is called a “VLDL remnant” and ultimately an IDL. Some (though not all) of the IDL particles undergo continued lipolysis to reduce in size and become the famous (or infamous) LDL particles. However, most of the IDL particles are actually cleared by liver LDL receptors and do not become LDL particles.
All along this process, the larger particles “shed” phospholipids and fatty acids and thus become cholesterol-rich. It is the LDL particle that is the ultimate delivery vehicle of cholesterol back to the liver in a process now called “indirect reverse cholesterol transport.” However, under certain circumstances the LDL will penetrate and deliver its cholesterol load to the artery walls. THIS IS EXACTLY WHAT WE DON’T WANT TO HAPPEN. (Sorry for the bold ALL CAPS – I know some of you may have fallen asleep by now, but I didn’t want anyone missing the punch line.) Because almost all cells in the body de-novo synthesize all the cholesterol they need, LDLs are not actually needed to deliver cholesterol to most cells.
The final important point I want to make about cholesterol transport is that it goes BOTH ways. Lipoprotein particles carry triglycerides and cholesterol from the gut and liver to the periphery (muscles and adipocytes – fat cells) for energy, cellular maintenance, and other functions like steroid creation (called “steroidogenic” purposes – remember the figure last week showing a cholesterol molecule and steroid molecule). Historically this process of returning cholesterol to the liver was thought to be performed only by HDL’s and has been termed reverse cholesterol transport, or RCT (you’ll need to subscribe — for free — to lecturepad.org to access this last link, which is well worth the time).
This RCT concept is outdated as we now know LDL’s actually perform the majority of RCT. While the HDL particle is a crucial part of the immensely complex RCT pathway, a not-so-well-known fact is that apoB lipoproteins (i.e., LDL’s and their brethren) carry most of the cholesterol back to the liver. In other words, the “bad” lipoprotein, LDL, does more of the cleaning up (i.e., taking cholesterol back to the liver) than the “good” lipoprotein, HDL!
The problem, as we’ll discuss subsequently, is that LDL’s actually do the bad stuff, too – they dump cholesterol into artery walls.
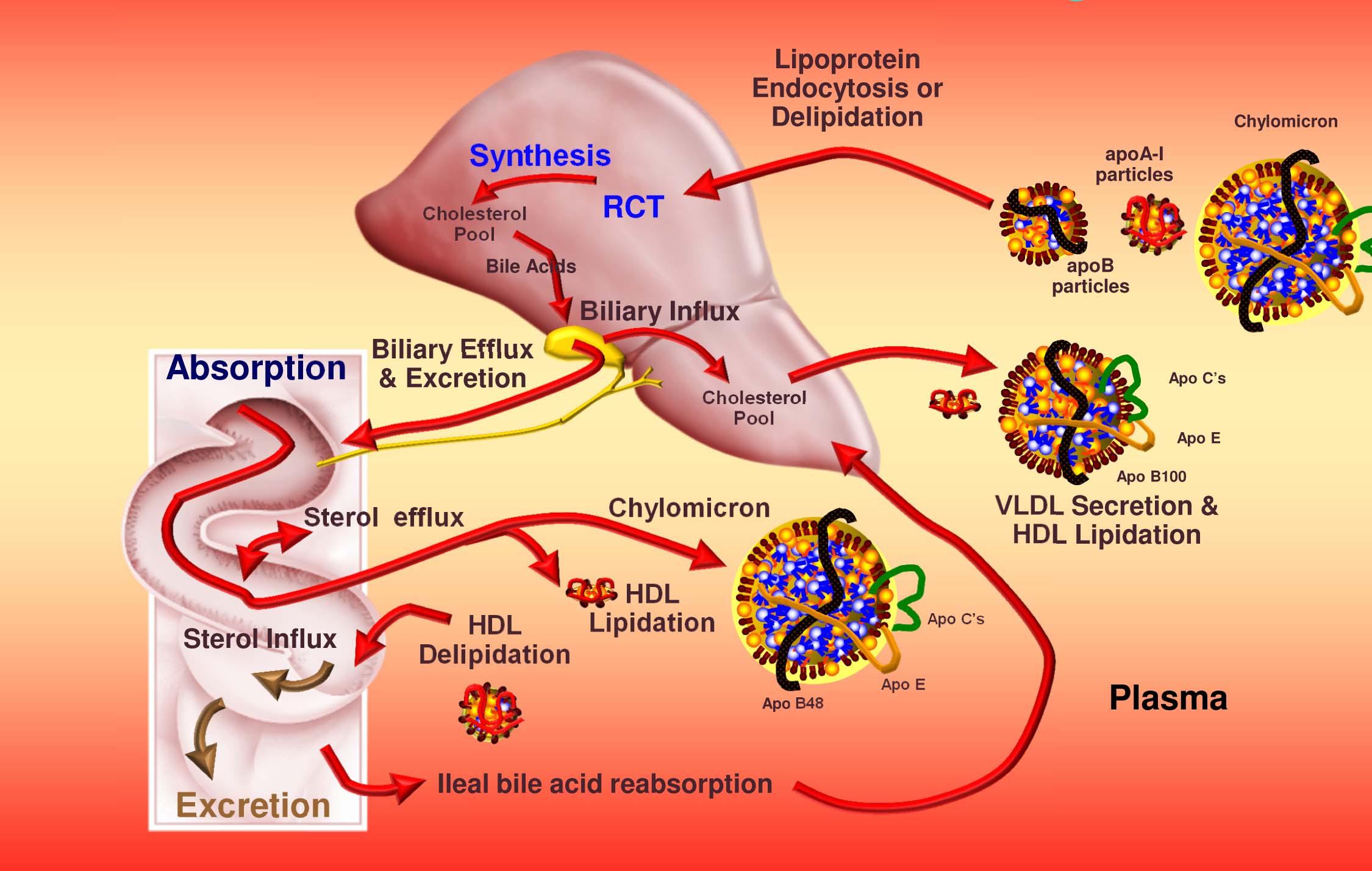
Let’s put this all together to summarize how cholesterol gets around our body
- Cholesterol and triglycerides are not soluble in plasma (i.e., they can’t dissolve in water) and are therefore said to be hydrophobic.
- To be carried anywhere in our body, say from your liver to your coronary artery, they need to be carried by a special protein-wrapped transport vessel called a lipoprotein.
- As these “ships” called lipoproteins leave the liver they undergo a process of maturation where they shed much of their triglyceride “cargo” in the form of free fatty acid, and doing so makes them smaller and richer in cholesterol.
- Special proteins, apoproteins, play an important role in moving lipoproteins around the body and facilitating their interactions with other cells. The most important of these are the apoB class, residing on VLDL, IDL, and LDL particles, and the apoA-I class, residing on the HDL particles.
- Cholesterol transport occurs in both directions, towards the periphery and back to the liver.
- The major function of the apoB-containing particles is to traffic energy (triglycerides) to muscles and phospholipids to all cells. Their cholesterol is trafficked back to the liver. The apoA-I containing particles traffic cholesterol to steroidogenic tissues, adipocytes (a storage organ for cholesterol ester) and ultimately back to the liver, gut, or steroidogenic tissue.
- All lipoproteins are part of the human lipid transportation system and work harmoniously together to efficiently traffic lipids. As you are probably starting to appreciate, the trafficking pattern is highly complex and the lipoproteins constantly exchange their core and surface lipids. This is a big reason why measuring how much cholesterol is within various lipoprotein species will in many circumstances be so misleading, as we’ll discuss subsequently in this series.
This was a bit of a tough one, so let’s stop there. Next week we’ll discuss how to actually measure cholesterol levels. In other words, if you’re looking at the river, with all its floating ships carrying their cargo, how do we measure the amount of cargo actually contained within the ships? Furthermore, is this the most important thing to be measuring? Ironically, it’s easier to measure the cargo in the ships, but more important to know the number of ships in the river. But now I’m getting ahead of myself.
P.S. Happy Birthday Dad (now I’ll know if you’re reading my blog!) [Originally posted on May 3, 2012]



